11-year-old boy with testicular pain and rash
An 11-year-old boy presented to the emergency department complaining of left testicular pain for 2 days, described as intermittent and stabbing, which ranged between 5 and 8 of 10 in intensity. Read the full case to see if you can correctly diagnose the patient.
Image credit | Author provided
THE CASE
An 11-year-old boy presented to the emergency department complaining of left testicular pain for 2 days, described as intermittent and stabbing, which ranged between 5 and 8 of 10 in intensity. The patient noted that his scrotum initially appeared red and swollen with the pain onset, which had since resolved. He had 1 episode of nausea and vomiting the day prior to evaluation when the pain was described as intractable. He otherwise denied fevers, diarrhea, trauma, hematuria, dysuria, sexual activity, or travel. A review of systems was notable for a pruritic rash on his ankles, which the patient presumed to be due to recent bug bites, with no other exposures being identified.
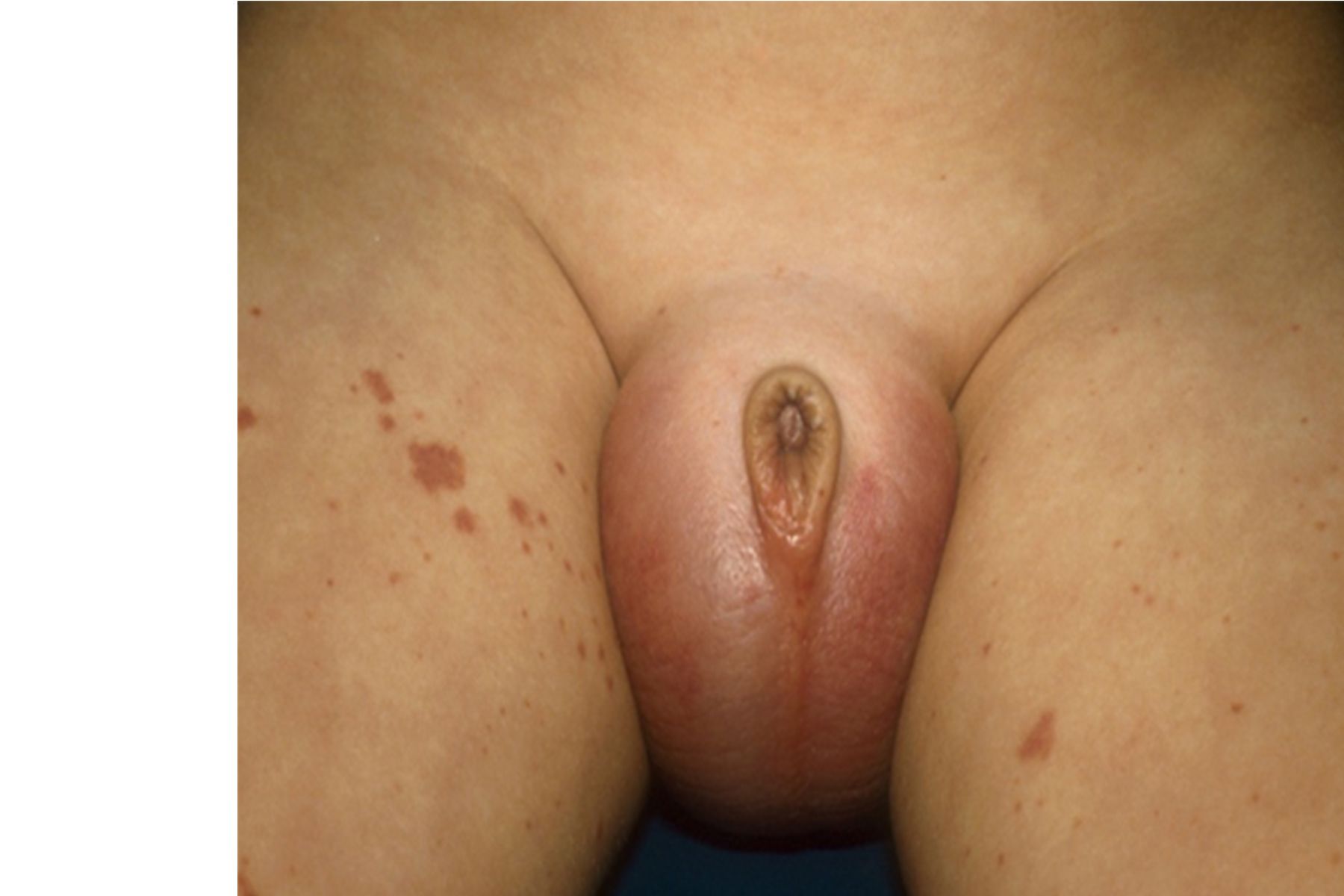
On examination, the patient was in mild distress due to pain, with age-appropriate vital signs. A genitourinary exam revealed a Tanner stage 1 circumcised male. Both testicles, left greater than right, were tender to palpation, not high riding, and with cremasteric reflexes present bilaterally; no masses, scrotal swelling, or erythema were appreciated. The left inguinal region was tender to palpation with no lymphadenopathy. A skin exam was notable for several scattered 2- to 3-mm blanching, erythematous macules and excoriations, in various stages of healing, located mainly on the ankles. The remaining findings of the physical exam were normal.
Diagnostic assessment included a Doppler ultrasound of both testes showing blood flow present and no thrombus bilaterally; however, the left testicle also showed heterogeneous and diffuse hypoechoic areas, consistent with subacute areas of ischemia or infarction, possibly suggesting a recent torsion-detorsion. A urinalysis result was normal, showing a specific gravity level of 1.013 and pH of 6.0, and being negative for leukocyte esterase, nitrates, blood, and protein. Results from a complete metabolic panel were also normal. A complete blood count revealed a total white blood cell count of 6600/µL (reference range, 3800-9800/µL), hemoglobin level of 10.9 g/dL (11.0-14.5g/dL), and platelet level of 278 x 103/µL (178-332 x 103/µL), with a normal differential.
The urology department was consulted and the patient proceeded to the operating room for emergent exploration, during which the left testicle was found to be mottled and ischemic, but blood flow was present, with no evidence of torsion. Of note, the right testicle additionally was found to have a similar mottled appearance to the left. A biopsy of the left testicle was taken and bilateral orchiopexy was performed.
Postoperatively, the previously noted ankle rash progressed to scattered areas of petechiae and nonblanching palpable 0.5- to 1-cm papules, some of which coalesced into crops of palpable purpura, extending onto the buttocks and posterior thigh region.
Differential diagnosis
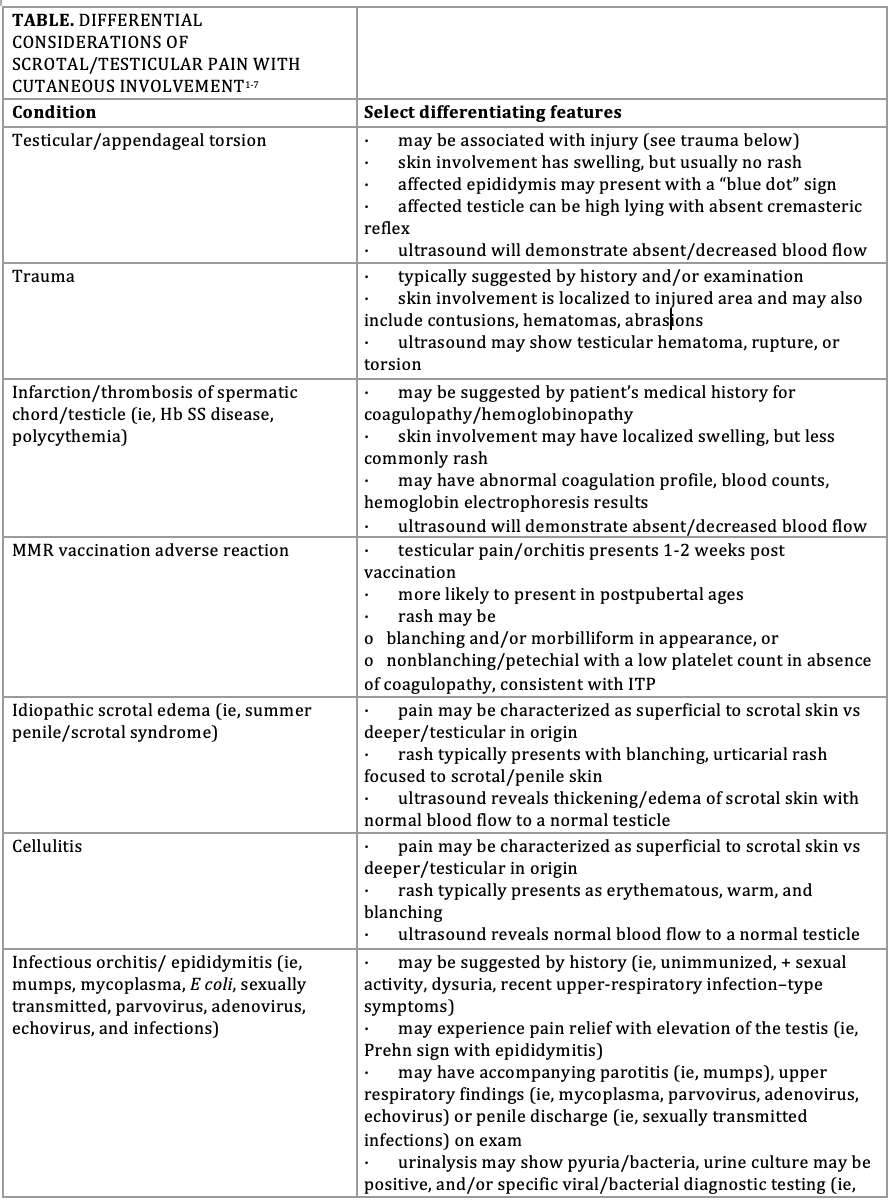
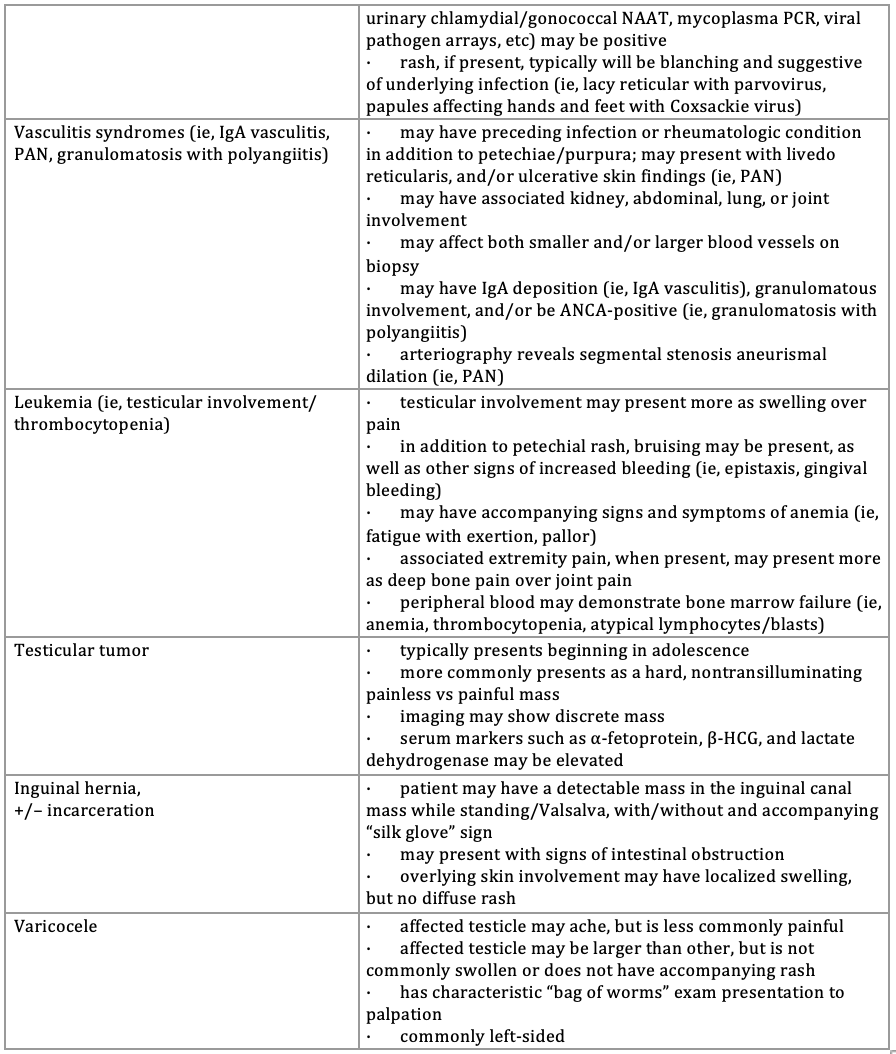
The Table1-7 lists differential considerations of scrotal or testicular pain in the presence of variable cutaneous findings, as occurred in this patient. Scrotal pain due to testicular torsion, a time-sensitive concern that should be corrected within 6 hours from pain onset to best ensure gonadal survivability, more commonly presents in boys older than 10 years with mostly unilateral, severe scrotal pain.1,2 Compared with the typical presentation, however, cremasteric reflexes were present in this patient’s presentation, and although scrotal swelling can occur, the accompanying rash was not typical. In addition, the operative findings of bilateral testicular involvement pointed toward a more systemic and/or tissue-targeted cause. A thrombus of the spermatic chord and/or testicle can also present similar to the patient’s presentation, but in this case had been ruled out by ultrasound.1,2 Other conditions presenting more with a blanching-type rash and scrotal pain may include idiopathic scrotal edema (ie, summer penile/scrotum syndrome); cellulitis; various infectious causes (see Table); and, more rarely, a reaction to the measles, mumps, and rubella (MMR) vaccination (ie, orchitis and/or morbilliform rash). However, rashes with both idiopathic scrotal edema and cellulitis are typically localized, and this patient had no history of recent MMR vaccination, nor other signs and symptoms at presentation pointing toward a more specific infectious etiology.1-5 Leukemia can cause a petechial/purpuric-type rash, as later presented in this patient, but scrotal involvement is typically more related to swelling and/or a discrete mass vs pain; additionally, a lack of systemic symptoms, thrombocytopenia, and indicators suggestive of other blood line involvement (see Table below) made an oncologic etiology a less immediate concern.2 Finally, characteristic evolution of the rash made vasculitis syndromes (ie, IgA vasculitis, polyarteritis nodosa) a consideration, prompting further history and evaluation below.
ANCA, antineutrophil cytoplasmic antibodies; E coli, Escherichia coli; HCG, human chorionic gonadotropin; Hgb SS disease, sickle cell anemia; ITP, idiopathic thrombocytopenia purpura; MMR, measles, mumps, rubella; NAAT, nucleic acid amplification test; PAN, polyarthritis nodosa; PCR, polymerase chain reaction.
Diagnosis
Further history revealed the patient had an episode of resolved sore throat 10 days prior to presentation and had been experiencing mild bilateral knee pain he forgot to mention in the context of his more intense testicular pain. An antistreptolysin O titer was performed, which came back elevated at 1005 IU/mL (< 200 IU/mL). Coagulation studies were also performed, whose findings were normal. Based upon the patient’s rash and preceding arthritis, in the context of normal results from platelet and coagulation studies, he received a diagnosis of IgA vasculitis (IgAV), also known as Henoch-Schönlein purpura.
Discussion
IgAV is the most common type of vasculitis in children, with peak incidence occurring during early school age, more commonly affecting boys.8,9 It is characterized by IgA immune-complex deposits in affected organ vessels, and although its cause is unknown, there is an association of IgAV developing after upper-respiratory viral or bacterial infections, such as group A Streptococcus pharyngitis, as occurred with this patient.9,10
Diagnosis is mostly based on clinical criteria agreed upon during the 2019 European Alliance of Associations for Rheumatology meeting, requiring presence of the characteristic palpable purpuric rash, in the absence of thrombocytopenia or coagulopathy, which is typically distributed in pressure and weight-dependent areas such as the lower extremities and buttocks. The diagnosis must also include one or more of the following, which may occur prior to, but more commonly follow, rash appearance: 1) nondeforming arthritis/arthralgia, 2) abdominal pain, 3) kidney involvement (ie, proteinuria, hematuria, nephritis), and/or 4) any organ biopsy showing an IgA vasculitis.10
Although patients with IgAV can more commonly present with the above findings, it is important to remember that scrotal pain, affecting the testicle(s) and/or epididymis, although not formally part of diagnostic criteria, is known to affect up to 38% of boys. It can manifest anytime during the condition’s course, even before the characteristic rash onset, as occurred in this patient, requiring increased suspicion for accurate diagnosis.1,6,11,12 Additionally, the pain-causing vasculitis that occurs within the testicle(s) can induce a torsion, so testicular involvement must be evaluated further by Doppler ultrasound to rule out torsion.1,13 A relative hypercoagulable state has also been hypothesized to contribute to tissue ischemia, as was observed in this patient, which should prompt further evaluation for infarction.12
Otherwise, rash is the presenting sign in approximately three-fourths of patients.14 The rash may begin as blanching, erythematous, macular, urticarial, or even targetoid in appearance, which typically evolves into petechiae and then palpable purpura.6,7,9,14 Subcutaneous edema is also known to variably occur.7 Finally, although not typical, the rash can initially present as pruritic.9 Therefore, depending on the stage of rash progression described above, differential considerations may include various infectious, allergic, autoimmune, thrombocytopenic, coagulopathic, and oncologic conditions.6,7,14 Joint pain, which occurs in over two-thirds of patients and commonly in larger, lower-extremity joints such as the knees and hips, may be accompanied by swelling; the pain is usually transient, but may be recurrent and severe enough that children will refuse to ambulate. Associated arthralgia and arthritis complaints may prompt differential considerations of trauma, septic joint, toxic synovitis, serum sickness, reactive arthritis, and other rheumatologic, and/or oncologic conditions, depending on the severity of pain, presence of swelling, and location and number of joints involved.6,7,14 Abdominal symptoms, experienced by over half of patients, range from pain to nausea to emesis in varying severity, with more severe symptoms progressing to gastrointestinal bleeding, intussusception, bowel ischemia, and perforation.6,7,14 In addition to the aforementioned conditions, abdominal pain can also variably present as acute mesenteric adenitis, gastroenteritis, pancreatitis, appendicitis, or constipation depending on its severity and location, the presence of vomiting, and other factors.6,7 Over one-third of patients develop urinary and kidney involvement with hematuria and/or proteinuria, with a smaller percentage developing glomerulonephritis or progressing to chronic kidney disease.1,6,7,14
IgAV is mostly a self-limited disease with initial management focused on symptomatic treatment and monitoring for complications. Approximately one-third of patients experience a recurrence, with smaller percentages (5%-20%) progressing to some of the aforementioned kidney, gastrointestinal, and other complications.6,7,14 For arthritis or arthralgia complaints, nonsteroidal anti-inflammatory drugs and symptomatic pain control are indicated.6,7,10,14 For abdominal pain, analgesia, fluid, and nutritional support may be required, as well as monitoring stool for gross blood or melena.6 Patients with severe abdominal pain and/or cases of gastrointestinal bleeding should receive an abdominal ultrasound to evaluate for any associated intussusception, ischemia, or perforation, with further treatment as appropriate.6,7,10,14 Steroid treatment to decrease the duration of severe abdominal pain refractory to other pain control and to prevent intussusception can be considered when more serious abdominal conditions are ruled out, but should be weighed against medication adverse effects.7,14,15 Additionally, although the strength of evidence is relatively weak, some literature and expert consensus suggest treating testicular pain with a short course of steroids; in unclear cases, however, surgical exploration still may be appropriate to exclude testicular torsion or other conditions.1,10,12 Blood pressure, weekly urinary function testing for blood and protein, plus monthly serum kidney function testing should be done for at least 6 months after diagnosis, regardless of initial results, to evaluate for nephritic, nephrotic, and/or chronic kidney disease.6,7,10 When such impaired kidney function concerns arise, specialist consultation is warranted for possible kidney biopsy and high-dose steroid/immunosuppressive therapy initiation.6,7,10,14
Patient course
Although immunofluorescence staining specific for IgA was not performed on the testicular biopsy tissue, it did show a small vessel leukocytoclastic vasculitis, consistent with IgAV.10,12,16 Postoperatively, the patient reported improvement in his scrotal pain, so given this improvement, he was not started on steroids. He had no further arthritis complaints but did develop some mild abdominal pain that was relieved with heat packs and supportive measures; he otherwise continued to eat and drink well, with no emesis, and stools remaining heme negative. The results of his blood pressure, urine, and kidney function studies remained normal during hospitalization. He was started on a course of amoxicillin for his recent streptococcal pharyngitis and discharged home, instructed to follow-up with his pediatrician for further urine and kidney function monitoring.
To view more Puzzlers, click here.
References
1) Dalpiaz A, Schwamb R, Miao Y, Gonka J, Walzter W, Khan SA. Urological manifestations of Henoch-Schonlein purpura: a review. Curr Urol. 2015;8(2):66-73. doi:10.1159/000365692
2) Kliegman RM, St. Geme JW III. Nelson Textbook of Pediatrics. 21st ed. Elsevier; 2019.
3) Clifford V, Wadsley J, Jenner B, Buttery JP. Mumps vaccine associated orchitis: evidence supporting a potential immune-mediated mechanism. Vaccine. 2010;28(14):2671-2673. doi:10.1016/j.vaccine.2010.01.007
4) McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1-34.10
5) Santi M, Lava SAG, Simonetti GD, Bianchetti MG, Milani GP. Acute idiopathic scrotal edema: systematic literature review. Eur J Pediatr Surg. 2018;28(3):222-226. doi:10.1055/s-0037-1603089
6) Kaminsky LW, Fletcher JP, Aprile JM. Case 3: abdominal pain and epididymitis in an 8-year-old boy. Pediatr Rev. 2017;38(9):438. doi:10.1542/pir.2016-0099
7) Leung AKC, Barankin B, Leong KF. Henoch-Schönlein purpura in children: an updated review. Curr Pediatr Rev. 2020;16(4):265-276. doi:10.2174/1573396316666200508104708
8) Barut K, Sahin S, Kasapcopur O. Pediatric vasculitis. Curr Opin Rheumatol. 2016;28(1):29-38. doi:10.1097/bor.0000000000000236
9) Du L, Wang P, Liu C, Li S, Yue S, Yang Y. Multisystemic manifestations of IgA vasculitis. Clin Rheumatol. 2021;40(1):43-52. doi:10.1007/s10067-020-05166-5
10) Ozen S, Marks SD, Brogan P, et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis–the SHARE initiative. Rheumatology (Oxford). 2019;58(9):1607-1616. doi:10.1093/rheumatology/kez041
11) Tabel Y, Inanc FC, Dogan DG, Elmas AT. Clinical features of children with Henoch-Schonlein purpura: risk factors associated with renal involvement. Iran J Kidney Dis. 2012;6(4):269-274.
12) Ma Y, Zhang S, Chen J, Kong H, Diao J. Henoch-Schönlein purpura with scrotal involvement: a case report and literature review. J Pediatr Hematol Oncol. 2021;43(6):211-215. doi:10.1097/MPH.0000000000002161
13) Stein BS, Kendall AR, Harke HT, Naiman JL, Karafin L. Scrotal imaging in the Henoch-Schönlein syndrome. J Urol. 1980;124(4):568-569. doi:10.1016/s0022-5347(17)55548-2
14) Du L, Wang P, Liu C, Li S, Yue S, Yang Y. Multisystemic manifestations of IgA vasculitis. Clin Rheumatol. 2021;40(1):43-52. doi:10.1007/s10067-020-05166-5
15) Weiss PF, Feinstein JA, Luan X, Burnham JM, Feudtner C. Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review. Pediatrics. 2007;120(5):1079-1087. doi:10.1542/peds.2007-0667
16) Brogan P, Eleftheriou D, Dillon M. Small vessel vasculitis. Pediatr Nephrol. 2010;25(6):1025-1035. doi:10.1007/s00467-009-1317-4
Newborn with midline neck lesion
December 21st 2023A 4-day-old boy with a midline neck lesion was born at term by normal vaginal delivery. After birth, mid line lesion had the configuration of a linear cleft with a cephalocaudal orientation, extending from the level below the hyoid bone to the suprasternal notch with a length of 2.5 cm and a width of 0.5 cm. What's the diagnosis?
A 13-year-old girl with well-demarcated rash on back and chest
October 19th 2023A healthy 13-year-old girl presented with a 1-month history of an asymptomatic, well-demarcated rash on her back and upper chest. The eruption consisted of discrete, dark brown papules that coalesced into large, flat-topped plaques with mild superficial scale and accentuation of skin markings. What's the diagnosis?
Suspicious facial swelling in a 22-month-old girl
October 11th 2023A 22-month-old female patient with sickle cell disease on folic acid and penicillin prophylaxis with a 3-day history of nasal congestion, rhinorrhea, fever and decreased oral intake presents to the emergency department (ED) for acute facial swelling noted when she woke up from a nap. What's the diagnosis?
Friction-induced blistering on a child’s feet
July 14th 2023You are called to the hospital nursery to evaluate a healthy full-term newborn boy who developed painful flaccid blisters and erosions on the tops of his feet and ankles shortly after birth. His mother had a history of similar recurrent skin lesions that healed with scarring. She also had oral and gastrointestinal tract involvement. What's the diagnosis?