Takeaways:
- Dermavant Sciences released positive efficacy and safety data for tapinarof cream 1% in treating atopic dermatitis (AD) in patients aged 2 years and older.
- Tapinarof cream, an aryl hydrocarbon receptor agonist, is a once-daily, steroid-free topical cream approved for plaque psoriasis in adults.
- The interim analysis of the ADORING 3 study, evaluating tapinarof cream 1% in AD patients for up to 48 weeks, showed 51.2% achieving complete disease clearance.
- An integrated analysis of the ADORING development program, including 711 patients from phase 3 trials, demonstrated continued efficacy beyond the 8-week treatment period.
- Overall, 73% achieved a Validated Investigator Global Assessment for AD score of 0 or 1, and nearly 81% achieved at least a 75% improvement in the Eczema Area and Severity Index.
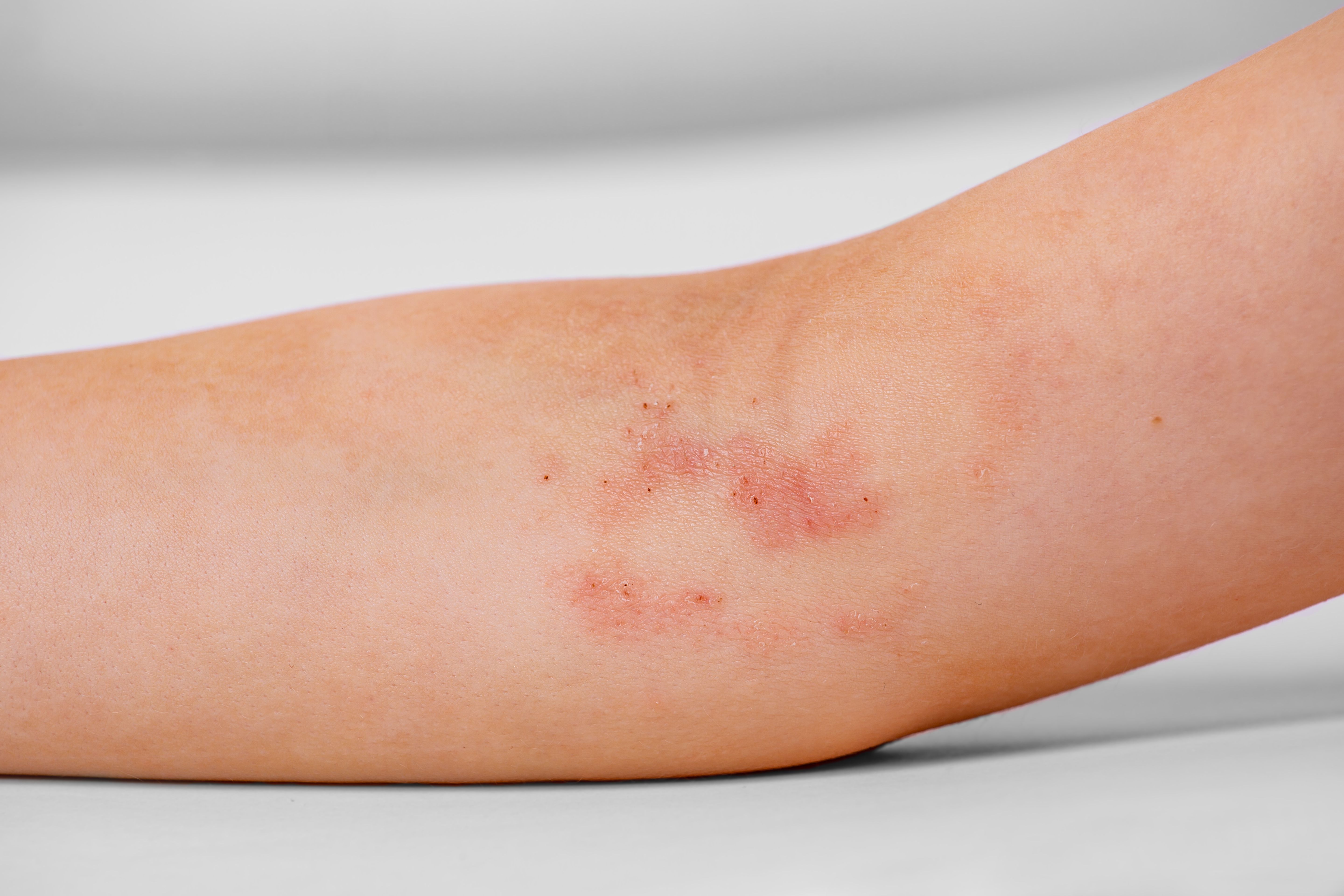
Dermavant Sciences has released new, topline, positive efficacy and safety data for tapinarof cream 1% (VTAMA) to treat atopic dermatitis (AD) in patients aged 2 years and older. The new data will be included in an expected supplemental New Drug Application (sNDA) submission during the first quarter of 2024.
The novel, aryl hydrocarbon receptor agonist is a once-daily, steroid-free topical cream in development for acute and long-term treatment/management of AD. Tapinarof cream 1% is currently FDA approved to topically treat plaque psoriasis in adults in the United States.
The data is from an interim analysis of the on-going ADORING 3 open-label, long-term extension study to evaluate the safety and efficacy of tapinarof cream 1% in AD patients for up to 48 weeks.
The company also completed an integrated analysis of data that included 711 patients from the identical phase 3 ADORING 1 (NCT05014568) and ADORING 2 (NCT05032859) trials, as well as the Maximal Usage Pharmacokinetics study for AD.
Results from the integrated analysis spanning the ADORING development program demonstrated that efficacy with tapinarof cream 1% continued beyond the 8-week double blind treatment period in ADORING 1 and ADORING 2. Click here for further details of these identical trials.
Overall, the integrated analysis revealed that 73% achieved a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 0 (clear) or 1 (almost clear), with at least a 2-grade improvement from baseline. Nearly 81% of patients achieved at least a 75% improvement in the Eczema Area and Severity Index (EASI75).
For patients aged 12 years and older, 77.9% with a baseline Peak Pruritus Numeric Rating Scale (PP-NRS) score of 4 or greater achieved a 4-point or greater reduction in PP-NRS. In the ADORING 1 and ADORING 2 studies, a mean itch reduction was observed as early as 24 hours after the initial application. Click here to read more about these results.
Interim analysis for ADORING 3 revealed that 51.2% achieved complete disease clearance (vIGA-AD score: 0).
“The integrated analysis data from the ADORING development program are particularly encouraging as they show a high level of efficacy in a diverse patient population, including patients down to 2 years of age, who are in need of a treatment option such as VTAMA cream that has demonstrated a positive safety and tolerability profile and potential long-term disease control in the ADORING studies,” said Eric Simpson, MD, MCR, professor, Frances J. Storrs Medical Dermatology, director, CLEAR Eczema Center, Oregon Health & Science University, Portland, Oregon, in a press release.
Simpson broke down the PP-NRS score in a October 2023 Q+A interview with Contemporary Pediatrics, click here for the details.
Simpson continued, “If approved for the treatment of atopic dermatitis, VTAMA cream’s efficacy and safety profile combined with its rapid onset of itch reduction, the most common symptom of atopic dermatitis, could provide an important new treatment option for not only patients suffering from the disease but also for their caregivers and the healthcare professionals treating them.”
In a December 2023 video interview with Contemporary Pediatrics, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, vice chair, Department of Dermatology, chief, Pediatric and Adolescent Dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego, California, discussed topical steroid and non-steroidal treatments for pediatric AD, and highlighted tapinarof cream 1% as a potential exciting treatment.
Click here to watch the full video interview.
Reference:
Dermavant announces positive data from the ADORING phase 3 development program in atopic dermatitis with VTAMA (tapinarof) cream 1% in adults and children as young as 2 years old. Dermavant Sciences. Press release. January 11, 2024. Accessed January 11, 2024. https://www.biospace.com/article/releases/dermavant-announces-positive-data-from-the-adoring-phase-3-development-program-in-atopic-dermatitis-with-vtama-tapinarof-cream-1-percent-in-adults-and-children-as-young-as-2-years-old/