Tapinarof cream 1% reduces itch as early as 24 hours after application for pediatric AD
A rapid reduction in pruritis as early as 24 hours after first application was announced as new positive data from a pair of identical, phase 3 studies of tapinarof cream 1% in children as young as 2 years and adults with atopic dermatitis (AD).
Tapinarof cream 1% reduces itch as early as 24 hours after application for pediatric AD | Image Credit: © Юля Шевцова - © Юля Шевцова - stock.adobe.com.
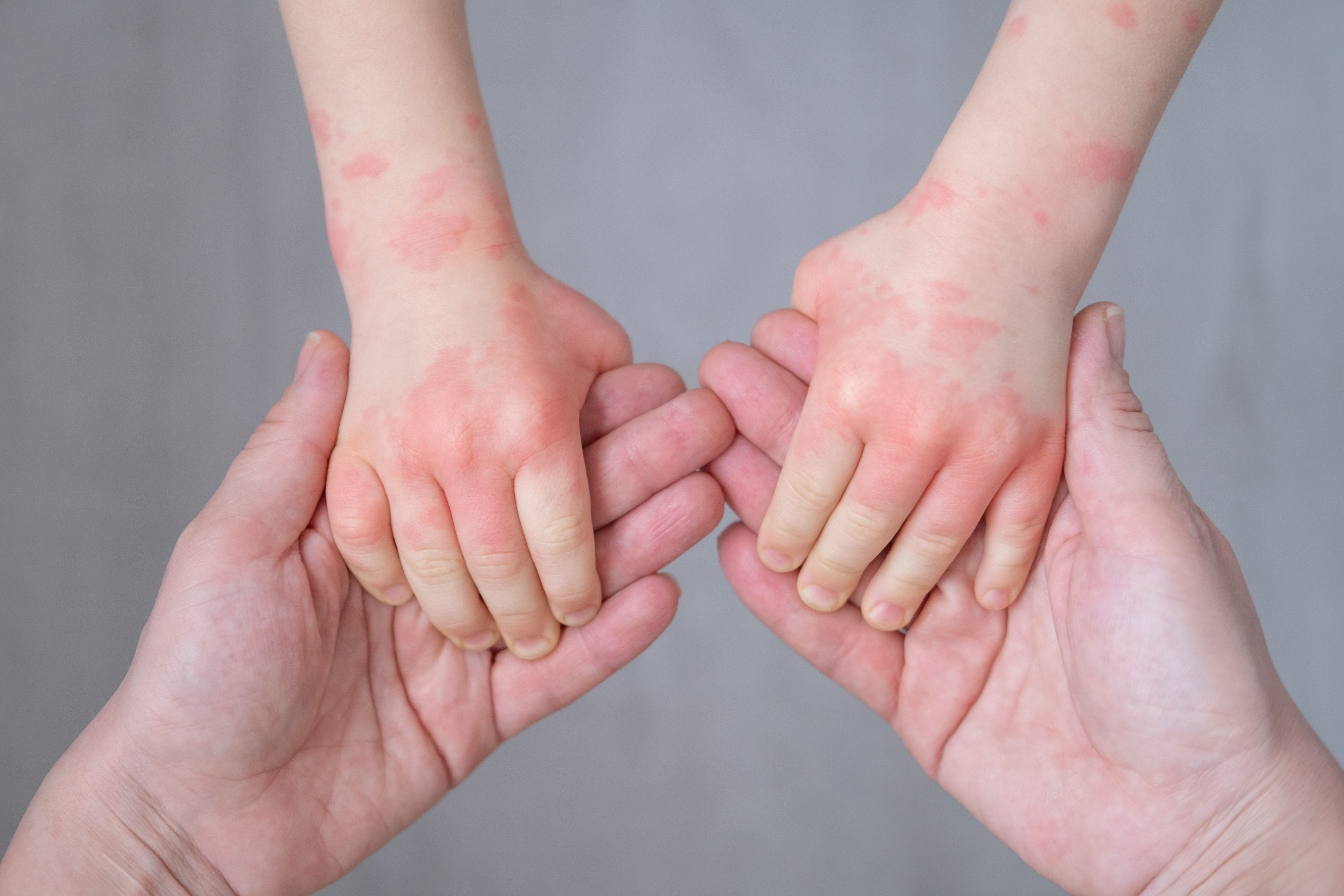
New pruritus data for tapinarof cream 1% (VTAMA; Dermavant Sciences) was announced at the European Academy of Dermatology and Venereology (EADV), revealing a greater reduction in itch as early as 24 hours after first application in children as young as 2 years of age with atopic dermatitis (AD), according to the announcement from Dermavant.
The new data was announced from 2 identical, double-blind, vehicle-controlled pivotal phase 3 trials, ADORING 1 (NCT05014568) and ADORING 2 (NCT05032859). Individuals in the trials were randomized 2:1 to tapinarof cream 1% or vehicle for 8 weeks. Mean changes in Peak Pruritus-Numeric Rating Scale (PP-NRS) evaluated itch relief efficacy endpoints. The PP-NRS considers a person’s worst itch over the previous 24 hours, evaluated on an 11-point scale with 0 indicating “no itch” and 10 indicating “worst imaginable itch.”
In a Q+A interview with Contemporary Pediatrics, study investigator Eric Simpson, MD, MCR, professor, Frances J. Storrs Medical Dermatology, director, CLEAR Eczema Center, Oregon Health & Science University, explains the recently-announced data, study details, and provides thoughts on an anticipated supplemental New Drug Application (sNDA) filing with the FDA for tapinarof cream 1% in the first quarter of 2024.
Contemporary Pediatrics:
Can you explain or break down the scoring of the PP-NRS scale, and how significant of a reduction in this score was observed in the study is?
Eric Simpson, MD, MCR:
The Peak Pruritus-Numeric Rating Scale (PP-NRS) is a widely used and validated tool in clinical studies of atopic dermatitis (AD) to measure the peak severity of itch experienced over a period of 24 hours. This scale ranges from 0 to 10, where 0 indicates no itch and 10 represents the worst imaginable itch. In these studies, itch relief was measured by mean changes in PP-NRS scores (daily and by visit on weeks 1, 2, 4, and 8) from baseline through week 8. The results are as follows:
- For daily evaluations of itch from baseline, greater reductions in PP-NRS mean scores for VTAMA cream versus vehicle were observed as early as Day 1 in ADORING 1 (-1.2 vs -0.9), 24 hours after first application, and Day 2 in ADORING 2 (-1.6 vs -1.4).
- Tapinarof achieved statistically significant and clinically meaningful reductions in mean weekly PP-NRS scores as early as week 1 compared with vehicle in ADORING 1 (-2.0 vs -1.2 [P < 0.0001]) and ADORING 2 (-2.0 vs -1.3 [P = 0.001]).
- Greater reductions in mean PP-NRS scores with VTAMA cream versus vehicle were seen for all visits through week 8 in ADORING 1 (-4.1 vs -2.6 [P < 0.0001]) and ADORING 2 (-4.1 vs -2.4 [P < 0.0001]).
Overall, these results show a rapid reduction in itch as early as 24 hours after first application of tapinarof, with a consistent improvement in itch through week 8. Itch remains the most burdensome and prevalent symptoms of atopic dermatitis, and these results highlight the potential of tapinarof as a clinically meaningful therapy with the ability to reduce itch for adults and children living with atopic dermatitis.
Contemporary Pediatrics:
How can the reduction in itch that was observed in the study as early as 24 hours change the treatment landscape of for AD? Can you provide details that were observed in regard to the timing of treatment? How often is it applied, and does tapinarof require continued treatment?
Simpson:
Itch remains the most burdensome symptom of atopic dermatitis, negatively impacting the quality of life of not only the patients but also their families. Itch has been implicated in the sleep disruption observed in patients with AD. Chronic sleep disturbance is thought to impacts patients’ mental health in adults and possibly neurodevelopment in children. Barriers to effective therapy in AD include poor adherence to topical therapy due to stinging and burning in nonsteroidal therapies, concerns about safety with topical steroids or calcineurin inhibitors which carry a boxed warning. I anticipate tapinarof becoming an effective therapy in the real-world because of the following novel characteristics: once daily application, cream formulation, lack of significant burning, rapid onset of potent anti-inflammatory and anti-itch effects. These new results showed rapid itch relief, as early as day 1 in ADORING 1 and day 2 in ADORING 2. While there are no head-to-head studies that compare tapinarof to other treatments, we are confident in tapinarof’s potential if approved— as a novel, steroid-free topical therapy — to become a safe, highly effective, and well-tolerated treatment option for adults and children as young as two years old with atopic dermatitis.
In these studies, tapinarof was applied once daily for 8 weeks. I look forward to learning about the long-term use of tapinarof in people with AD in our ADORING 3 study. In the psoriasis trials, some patients experienced a prolonged period of clearance after stopping tapinarof and we will certainly be evaluating this in the long-term extension studies.
Contemporary Pediatrics:
Regarding the anticipated sNDA filing with the FDA, how important could a potential approval of tapinarof cream 1% be for the pediatric population, and what other treatments are there for this age indication?
Simpson:
Typical treatment for children with atopic dermatitis includes topical steroids and topical calcineurin inhibitors that both have significant limitations due to safety and tolerability. Potent and safe topical therapies for atopic dermatitis remain an area of unmet medical need, particularly in children. Caregivers for children with atopic dermatitis often seek topical, non-steroidal options that are safe and offer long-lasting relief. However, current topical treatment options may not be able to be used long term for a chronic disease, due to limitations about where they can be used on the body and for how long, and some of these treatments, if used over an extended period of time, may have serious, sometimes irreversible side effects. The potential approval of tapinarof could be a significant development in the treatment of atopic dermatitis. If approved, tapinarof could provide another much-needed treatment option for patients with atopic dermatitis, particularly those who seek a non-steroidal option.
Contemporary Pediatrics:
Overall, what are your thoughts on the announcement of this new data, and how does it play a role in the overall development of tapinarof cream 1%?
Simpson:
Given that itch remains the most burdensome and prevalent symptom of atopic dermatitis, I’m excited by these results which underscore the potential of tapinarof to be a therapeutic option with the ability to reduce itch for adults and children as young as 2 years old. Building upon the topline results from ADORING 1 and 2, these findings suggest tapinarof has the potential to advance the treatment landscape for adults and children with atopic dermatitis through disease control and symptom reduction capabilities.
Tapinarof has already brought changes to the plaque psoriasis treatment landscape – [tapinarof] cream is already approved for the treatment of plaque psoriasis in adults. In the 2 months following approval, I believe tapinarof cream became the most prescribed branded topical treatment for plaque psoriasis in adults. I look forward to learning more about how tapinarof can bring about change for those with atopic dermatitis, given that nonsteroidal options that can meet patients’ expectations for safety and efficacy are sorely needed in this space.
Reference:
Dermavant announces new positive pruritus data for VTAMA (tapinarof) cream, 1% in adults and children as young as two years old with atopic dermatitis at the EADV Congress 2023. Dermavant Sciences. Press release. October 12, 2023. Accessed October 12, 2023.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.
Young woman with tick bites presents with erythematous papules, headaches, and fatigue
April 8th 2024A young woman with no significant past medical history returns from hiking with several white-spotted ticks and experiences erythematous papules, rashes, headaches, and fatigue. What’s the diagnosis?