Office rapid strep tests: State of the art
When I started my pediatric practice in 1986, we tested patients for strep throat by performing a throat culture, which was placed in a small office incubator for 48 hours. Typically, we put patients on an antibiotic pending culture results and would stop antibiotics if the culture proved negative. In my first year of practice, an interesting new technology arrived-rapid antigen detection tests (RADTs). These tests were reasonably accurate and enabled us to make a diagnosis at the time of the visit.
When I started my pediatric practice in 1986, we tested patients for strep throat by performing a throat culture, which was placed in a small office incubator for 48 hours. Typically, we put patients on an antibiotic pending culture results and would stop antibiotics if the culture proved negative. In my first year of practice, an interesting new technology arrived-rapid antigen detection tests (RADTs). These tests were reasonably accurate and enabled us to make a diagnosis at the time of the visit.

Rapid diagnostics have advanced amazingly over the past few decades. In this installment of Peds v2.0, we will look at what is state-of-the-art in terms of rapid diagnosis of strep pharyngitis.
Strep pharyngitis: The nuances
In the United States there are 15 million visits to primary care physicians for pharyngitis each year.1 Twenty percent to 30% of pharyngeal infections in children are attributed to group A streptococcus (GAS), also known as Streptococcus pyogenes. In contrast, only 5% to 15% of throat infections in adults are caused by GAS.2
Recommended: When rash and fever become an emergency
In distinguishing strep pharyngitis from viral infections, one needs to focus on the symptoms and signs in the presenting patient. Strep infections are not accompanied by symptoms typical of a viral illness, ie, cough, ulcers on the pharynx, and rhinorrhea. Symptoms of strep pharyngitis often include fever, enlarged and painful cervical nodes, sore throat, often headache, as well as abdominal pain with or without nausea and vomiting.3 Signs often associated with strep infections are tonsillar inflammation or exudate, petechial on the soft palate, and enlarged tender lymph nodes.
Some pediatricians use the “strep score” to help identify strep pharyngitis. This score was first developed by Centor and later modified by McIsaac to take the patient’s age into consideration. If you use the McIsacc score, patients are assigned 1 point each if: 1) they are aged between 3 and 14 years; 2) do not have a cough; 3) have swelling or exudate of the tonsils; 4) have tender anterior cervical nodes; or 5) have a fever exceeding 38°C (100.4° F). A score of 0 or 1 indicates that strep is unlikely and no strep test should be performed. A score of 4 or 5 indicates that a strep infection is more likely and strep testing should be performed. Only half of patients with a high strep score prove to have a positive culture.4,5
Also complicating the diagnosis of strep pharyngitis is that 15% to 20% of children are “carriers” of strep in the oropharynx and do not get strep infections, but present with a viral pharyngitis, test positive for strep, and receive antibiotic treatment.3 Carriers of strep do not manifest an immune response to strep that would be expected in infection. Antibody testing (anti-deoxyribonuclease B, antistreptolysin O) can distinguish carriage from true infection in suspected cases.
Treatment of strep infections with penicillin or alternative antibiotics shorten the course of the infections slightly; prevent suppurative complications such as peritonsillar abscess or lymphadenitis; prevent rheumatic fever and rheumatic heart disease; and reduce the spread of the illness in schools and households. However, rheumatic fever is very rare in first world countries with an incidence of less than 1 per 100,000 infections. In other parts of the world, rheumatic fever continues to be a major cause of heart disease in children, so the approach to the diagnosis and treatment of strep can vary by country.3
NEXT: How diagnosing strep has changed
Diagnosing strep: Then and now
Before the availability of RADTs in the 1980s, pediatricians employed several methods of testing swabs for strep. Studies found that using a 2-swab sample is more reliable in detecting strep compared with a single swab. Additionally, placing the swab in Todd Hewitt broth for 2 hours before plating often increases the likelihood of generating a positive culture, especially when there are few bacteria on the throat swab.3
More: 2015 acute otitis media update
In the 1980s, latex agglutination tests were the first commercially available tests to speed the diagnosis of strep infections. Specificity was excellent (false positive rate was low), but sensitivity was often poor (false negative rate was high), so negative tests were always backed up with a throat culture. These were eventually replaced by enzyme linked immunoassays.
The most popular rapid strep tests on the market today are lateral flow immunoassays. In this test, antibody specific to GAS carbohydrate cell wall antigen is coated on the test line region of a test strip or membrane. During testing, the extracted throat swab specimen reacts with an antibody to GAS that is coated onto particles. The mixture migrates up the strip or membrane to react with the antibody to GAS on the strep and generate a colored line in the test region. The presence of this line in the test region indicates a positive result, whereas its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control region if the test has been performed properly. If a control line does not appear, the test result is not valid. Keep in mind that false negative tests are associated with poor specimens, uncooperative patients, inexperience, or testing a patient early in the course of a strep infection.
NEXT: Top of the line strep tests
The latest and greatest strep tests
Although it still takes labs 48 hours to process throat cultures, many clinical labs are now able to perform polymerase chain reaction (PCR) testing for strep! Rather than testing for the cell wall antigen of the strep bacteria, PCR tests for the nucleic acid sequences associate with genes unique to the S pyogenes bacteria.
In many situations, sensitivity and specificity of PCR compared with culture are 100%. In 1 study, PCR testing of strep nucleic acid sequences was so exquisitely sensitive, the technique had a “limit of detection” of just 1 or 2 strep bacteria!6 Best of all, PCR takes about an hour to perform and costs less than $3 per test.7
Recommended: Communicating in the high-tech office
Several new rapid strep tests have appeared on the market over the past few years. These utilize a Clinical Laboratory Improvement Amendments (CLIA)-waived device to improve detection of S pyogenes. All have improved sensitivity over traditional lateral flow immunoassay testing.
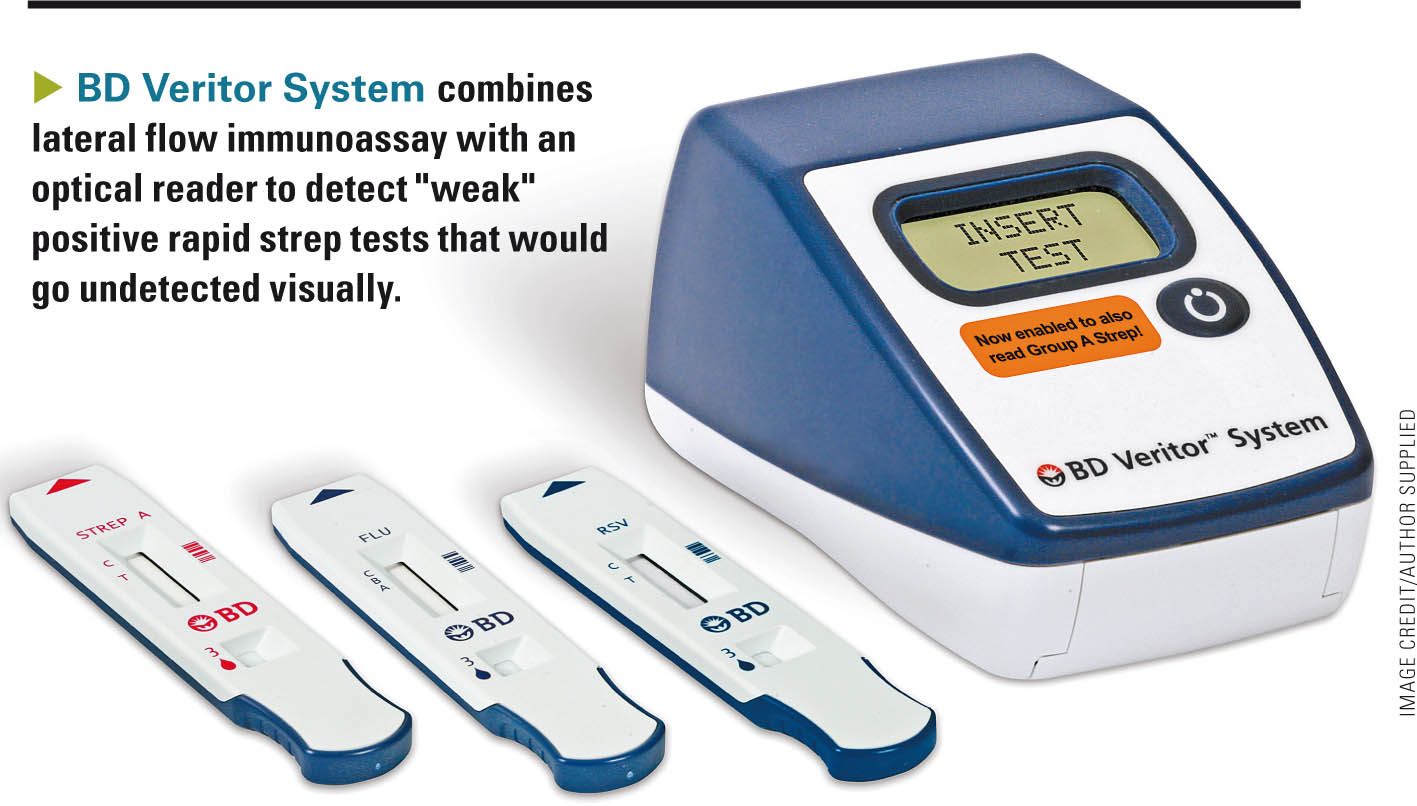
The BD Veritor System from BD Diagnostics (Franklin Lakes, New Jersey), combines a lateral flow immunoassay with an optical reader to detect “weak” positive rapid strep tests that would otherwise go undetected visually. The reader costs $300, and the tests sell for $4 to $6 per test. Reimbursement by most insurance companies varies from $10 to $16 per test. The test uses a 2-reagent extraction step with which most pediatricians are familiar. Three drops of extracted specimen are placed in the sample well in the cartridge. After 5 minutes, the cartridge is inserted into the optical reader, which then displays the results. Most traditional lateral flow immunoassay tests have a “limit of detection” of 105 to 107 colony-forming units per mL (cfu/mL).8 The Veritor strep test has a threshold of 104 to 105 cfu/mL (package insert).
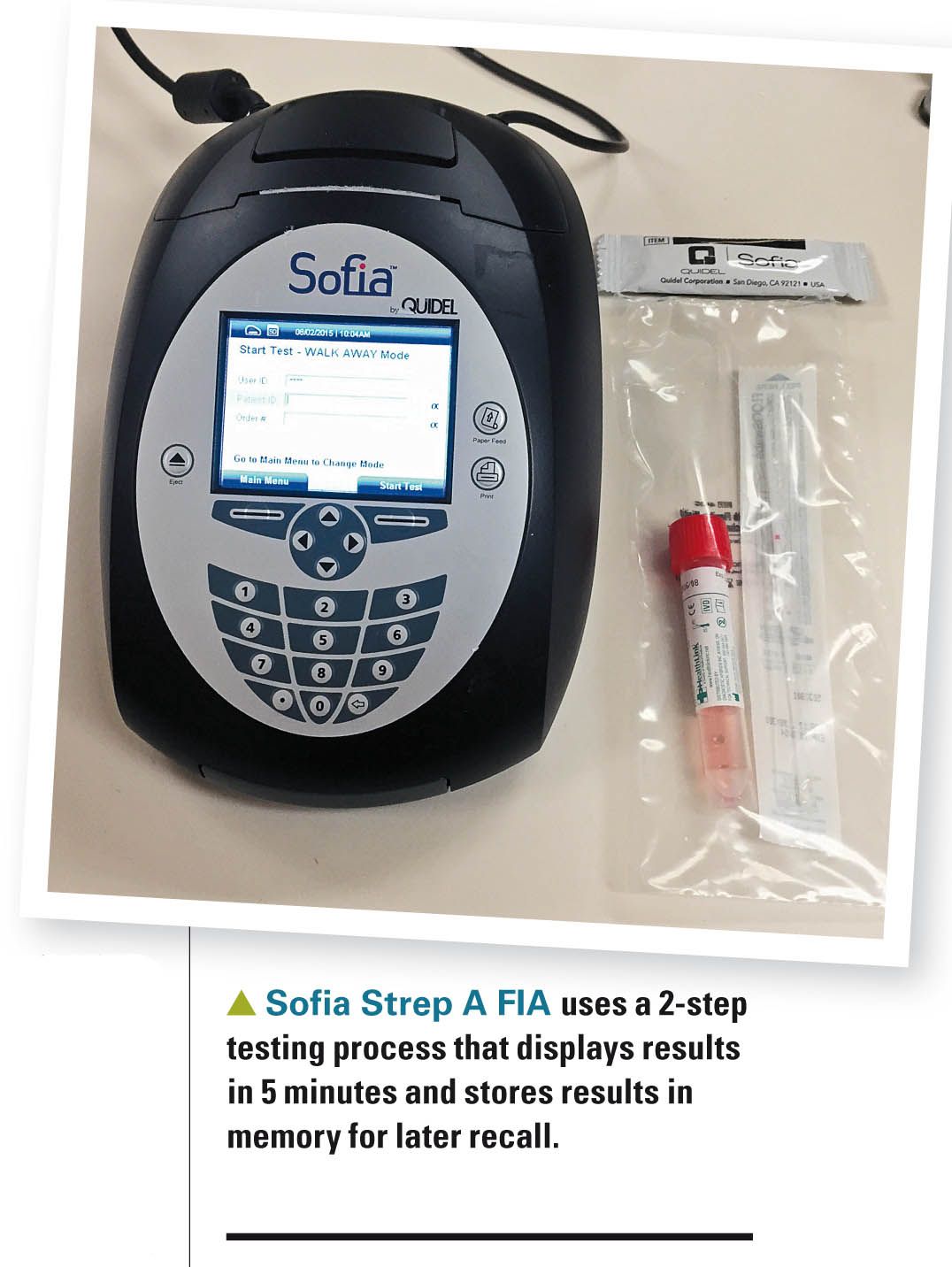
The Sofia Strep A FIA (Quidel; San Diego, California) involves an extraction and testing phase. Following a 1-minute extraction from a throat swab, an aliquot of the specimen is dispensed into a testing cassette sample well. From the sample well, the specimen migrates through a test strip containing various “chemical environments.” If GAS antigens are present, they will be bound by antibodies coupled to fluorescent microparticles that migrate through the test membrane. The fluorescent microparticles containing bound antigen will be captured by antibodies on the test membrane where they are detected by the Sofia device. If antigens are not present, the fluorescent microparticles will not be trapped by the capture antibodies nor detected by the Sofia.
What’s nice about the Sofia test is that it features a “walkaway mode,” so once the test cartridge is inserted and the test started, the device displays a result in 5 minutes and prints results via an integrated printer. All results are stored in memory, and results can be recalled with the press of a button. The limits of detection of the test are 103 to 104 cfu/mL. The reader costs $4500 and each tests costs about $10.
NEXT: Molecular strep testing
Waived “molecular testing” is now available
Alere (Waltham, Massachusetts) gained CLIA-waived status in April 2015 for its i Strep A test system. The i Strep A device detects GAS bacteria in throat swab specimens using Alere's proprietary isothermal nucleic acid amplification technology that produces results within 10 to 15 minutes. A color video displayed on the device’s screen walks users through the testing process. A sample receiver cartridge is placed in the machine and warmed for 3 minutes. The swab is placed in the cartridge for just 10 seconds, and then a pipette cartridge is used to transfer the sample onto a testing cartridge. The lid is closed and the testing cycle begins.
More: Best tech for Pediatrics

You can walk away at this point and return in 8 minutes to see the result on the device’s LCD screen. Results are stored in memory and can be printed via an attached printer. According to Alere, the overall sensitivity and specificity of the Alere i Strep A are 95.9% and 94.6%, respectively. Alere could not tell me the device’s “limit of detection,” but because it is a PCR-type test one would expect it to be low (see above). The test is a “molecular test” so it is billed under a unique CPT code- 87651-that yields higher reimbursement compared with nonmolecular strep tests that are billed under the code 87880QW. The cost of the Alere i Strep A device and tests have not been released as of this writing.
NEXT: When are backup cultures needed?
Closing thoughts
I am amazed how strep testing has evolved over the years. We now have several new device-based strep tests that improve our ability to detect GAS early in the course of an illness or with a limited sample. I am especially excited that PCR-type technology has now achieved CLIA-waived status. No doubt other waived “molecular” tests will be available in the near future that will improve our ability to identify a wide spectrum of infectious agents at the time of the office visit, expediting the diagnosis and treatment of frequently encountered pediatric illnesses.
Next: What's new for auscultation?

REFERENCES
1. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA. 2000;284(22):2912-2918.
2. Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17(3):571-580.
3. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):1279-1282.
4. Dunne EM, Marshall JL, Baker CA, et al. Detection of group A streptococcal pharyngitis by quantitative PCR. BMC Infect Dis. 2013;13:312.
5. Cohen JF, Chalumeau M, Levy C, et al. Spectrum and inoculum size effect of a rapid antigen detection test for group A streptococcus in children with pharyngitis. PLoS One. 2012;7(6):e39085.
6. Thenmozhi R, Balaji K, Kanagavel M, Karutha Pandian S. Development of species-specific primers for detection of Streptococcus pyogenes from throat swabs. FEMS Microbiol Lett. 2010;306(2):110-116.
7. Slinger R, Goldfarb D, Rajakumar D, et al. Rapid PCR detection of group A streptococcus from flocked throat swabs. Ann Clin Microbiol Antimicrob. 2011;10:33.
8. Charlier-Bret N, Boucher B, Poyart C, et al; French health products safety agency (Afssaps). Rapid antigen detection tests for diagnosis of group A streptococcal pharyngitis: comparative evaluation of sensitivity and practicability of 16 in vitro diagnostics medical devices performed in July 2002 by the French health products safety agency (Afssaps) as part of its market control mission [article in French]. Pathol Biol (Paris). 2004;52(8):438-443.
Dr Schuman, section editor for Peds v2.0, is adjunct assistant professor of pediatrics, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire, and editorial advisory board member of Contemporary Pediatrics. He has nothing to disclose in regard to affiliations with or financial interests in any organizations that may have an interest in any part of this article.
Anger hurts your team’s performance and health, and yours too
October 25th 2024Anger in health care affects both patients and professionals with rising violence and negative health outcomes, but understanding its triggers and applying de-escalation techniques can help manage this pervasive issue.