Teen With Multiple Boils
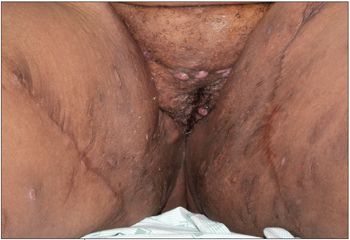
This 17-year-old presented with multiple boils in the perineum and under breast and skin folds. The lesions produced a malodorous discharge (which caused problems with peer acceptance at school) and were increasing in size. Oral antibiotics had not helped. The patient was admitted for intravenous antibiotic therapy.
HISTORY
This 17-year-old presented with multiple boils in the perineum and under breast and skin folds. The lesions produced a malodorous discharge (which caused problems with peer acceptance at school) and were increasing in size. Oral antibiotics had not helped. The patient was admitted for intravenous antibiotic therapy.
This patient has experienced previous similar episodes, in which lesions were largely confined to the perineum. Other areas are now involved, and the outbreaks have been occurring with increasing frequency.
Earlier outbreaks were treated with a short course of antibiotics, which afforded temporary relief. Some lesions were incised and drained.
PHYSICAL EXAMINATION
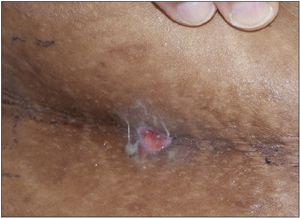
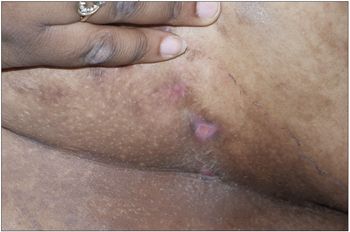
Obese patient; body mass index, 52.2 (above the 95th percentile). Nodulocystic swellings 1 to 1.5 cm in diameter in the perineum, axilla, and under mammary folds and skin folds on the back. Some swellings drained yellowish, foul-smelling purulent discharge, which was collected for culture. Hypertrophic granulation tissue was noted in some of the draining lesions. The patient had hyperpigmented areas and scars from surgery and old healed lesions.
LABORATORY EVALUATION
White blood cell count: 9400/µL, with 76% neutrophils, 21% lymphocytes, and 3% monocytes. Hemoglobin, 11.3 g/dL, with evidence of microcytic, hypochromic anemia. Serum electrolyte values, normal. Random blood glucose, 139 mg/dL. Serum levels of free insulin, 174 µIU/mL; total insulin, 284 µIU/mL; free testosterone, 0.55 ng/dL. Bioavailable testosterone levels elevated at 13.5 ng/dL. Insulin antibodies not detected. Normal levels of thyroid hormones, free thyroid-stimulating hormone, sex hormone-binding globulin, cortisol, dehydroepiandrosterone sulfate, follicle-stimulating hormone, somatomedin C, and serum albumin.
Culture grew a few coagulase-negative staphylococci and Corynebacterium species. A staphylococcal survey of the patient was negative, as were results of a urine pregnancy test.
WHAT'S YOUR DIAGNOSIS?
ANSWER: HIDRADENITIS SUPPURATIVA
Hidradenitis suppurativa (HS) is derived from the Greek word "hidro," meaning sweat, and "aden," meaning gland. The condition (also referred to as acne inversa or Verneuil disease) involves a chronic, suppurative inflammation of the apocrine glands in the perineum, axilla, and submammary and perianal regions.
The pathological process in HS involves initial occlusion of hair follicles. Sweat accumulates beneath keratin plugs and promotes bacterial proliferation. The most common causative microorganism is Staphylococcus aureus.1 The resulting inflammation gradually increases, resulting in the recruitment of neutrophils, macrophages, and other phagocytic cells to the site. This cellular response coupled with the release of inflammatory mediators leads to a breakdown of the walls of the hair follicles and spread to surrounding tissues. The inflammation eventually can lead to formation of abscesses that rupture and heal, leaving behind scars and deep sinuses.
EPIDEMIOLOGY
HS affects both men and women. For unknown reasons, however, women are more often affected. Danish studies show that females are affected 3 times as often as males.2 In men, the perianal region tends to be involved; in women, the perineal region and axilla are usually affected.3 African American women are often affected with this disease and are at high risk for scarring.4
HS typically begins during puberty (between 11 and 14 years) and continues throughout life, with episodes of flaring and quiescence. However, it can develop at any time: rare cases that developed before puberty and after menopause have been recorded.
PREDISPOSING FACTORS AND ASSOCIATIONS
The precise cause of HS has not been determined, but numerous factors are known to be associated with the disease. Studies done in 3 English families suggest that an autosomal dominant pattern of inheritance is operative in at least some families.3 Studies have also shown a greater likelihood of a positive family history in women with HS than in men.5
The patient in our case had a strong family history of HS. Her mother and several maternal cousins were similarly affected.
An association between elevated androgen levels and HS has long been proposed.5 Studies have shown evidence for and against this association. Earlier studies mention that higher levels of free testosterone and lower levels of sex hormone-binding globulin are found in some women with this disease.5 More recent studies conducted in age and weight-matched controls failed to demonstrate such a relationship.6 However, a significant number of patients show evidence of hyperandrogenism in their biochemical profile (as does our patient), and antiandrogen drugs are one of the first-line agents in the treatment of this disease. Many affected women are obese, which may contribute to higher androgen levels. Premenstrual androgenic hormonal spurts are associated with exacerbation of disease.5,7
Immunological studies have demonstrated lower levels of T lymphocytes and increased frequency of HLA antigens A1 and B8 in patients with HS.8Cigarette smoking is believed to be a triggering factor for the disease.9 An association with Crohn disease has been reported in patients with HS; histology of cutaneous lesions in HS patients reveals foreign body-type granulomas in the skin that are similar to those seen in Crohn disease.10,11
DIAGNOSIS
Diagnosis of HS is based mainly on clinical suspicion along with a history of recurrence of similar lesions in regions like the axilla and perineum that are rich in apocrine glands. There may be a history of eruptions among family members. Biopsy is not needed but may be done in special cases when the diagnosis is difficult or when Crohn disease is suspected.
The differential diagnosis for HS includes Fox-Fordyce disease, recurrent folliculitis, and Crohn disease.
Laboratory investigations are not necessary, but a number of associated conditions--obesity and insulin resistance among them--may coexist. Thus, a screen for associated diseases is warranted. Blood investigations usually reveal relatively high levels of free androgens and low levels of sex hormone-binding globulin.5,6
MANAGEMENT
Therapy for patients with HS is multidisciplinary. Most patients eventually need both medical and surgical management. Treatment modalities can be described in 2 phases.
The acute phase. During an acute flare-up, treatment is focused on reducing the degree of inflammation.
Antibiotics. Clindamycin is the drug of choice in this setting. Other antibiotics such as minocycline and ciprofloxacin can also be used. Prolonged use of antibiotics may help prevent recurrences, but lesions reappear when therapy is discontinued. Keep in mind, however, that frequent use of broad-spectrum antibiotics can lead to antibiotic resistance. Also, there is no evidence or clinical study that demonstrates the usefulness of long-term antibiotic treatment.
Surgery. Incision of abscesses and unroofing of sinus tracts may be needed in some cases. Surgical intervention is required only when disease is severe; surgery cures only local disease and does not reduce the risk of recurrence at other sites.
The chronic phase. Long-termtherapy focuses on preventing new lesions and on treating possible exacerbating factors.
Antiandrogens. These agents have long been used to treat HS. Oral contraceptive pills that contain progestins with low androgenic potential have been used with promising results.12 Cyproterone acetate, with its antitestosterone effect on tissues, has been shown to decrease the number of recurrences.13 Other antiandrogens used include spironolactone and finasteride.14
Retinoids. Isotretinoin (0.5 to 1 mg/kg/d) has been used to treat severe disease and to reduce the inflammatory response before surgery.15 Retinoids appear to act by reducing abnormal keratinization and by decreasing sebum production. The risk of adverse effects-such as liver toxicity, pseudotumor cerebri, skin and hair changes, and teratogenic potential-often limit long-term use of this therapy.
Suppression of tumor necrosis factor. TNF-α is an important mediator of inflammation; it acts by increasing the release of acute phase reactants from the liver and is a potent activator of neutrophils and macrophages. Infliximab (Remicade) is a chimeric mouse-human monoclonal antibody to TNF-α. Adalimumab (Humira) is a human monoclonal antibody to TNF-α. Etanercept (Enbrel) is a fusion protein that resembles the TNF-α receptor. All these drugs can bind to and prevent the action of TNF-α, thereby decreasing the inflammatory response in patients with HS.16-18
Cyclosporines. These agents are commonly used as immunosuppressants. Cyclosporin A is the most commonly used drug in this class. The ability of these agents to inhibit the release of inflammatory mediators by T lymphocytes helps control frequent flares of HS.19 The risk of such adverse effects as renal and hepatotoxicity limit their use, however.
Surgery. Wide excision along with reconstructive flaps as well as skin grafts can greatly improve the quality of life for patients with severe debilitating disease. In some instances, carbon dioxide laser surgery has been used to obliterate the apocrine glands in select areas of the body with good results.20-22
Lifestyle measures. Maintenance of personal hygiene, smoking cessation, weight reduction, and control of coexisting conditions (eg, diabetes) need to be addressed.
OUTCOME OF THIS CASE
The patient was treated with intravenous clindamycin (600 mg q8h) for 48 hours. Hypertrophic granulation tissue was cauterized with silver nitrate. After hospital discharge, the patient took oral clindamycin (450 mg) for 10 days. She was to continue therapy with oral ciprofloxacin (250 mg bid) after completing the clindamycin course.
By the eighth day of oral clindamycin therapy, all skin lesions were healing. No new lesions had appeared.

References:
REFERENCES:
1.
Lapins J, Jarstrand C, Emstestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery.
Br J Dermatol.
1999;140:90-95.
2.
Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions.
J Am Acad Dermatol.
1996;35(2, pt1):191-194.
3.
Fitzsimmons JS, Fitzsimmons EM, Gilbert G. Familial hidradenitis suppurativa: evidence in favour of single gene transmission.
J Med Genet.
1984;21:281-285.
4.
Melkun ET, Few JW. The use of biosynthetic skin substitute (biobrane) for axillary reconstruction after surgical excision for hidradenitis suppurativa.
Plast Reconstr Surg.
2005;115;1385-1388.
5.
Jemec JB. The symptomatology of hidradenitis suppurativa in women.
Br J Dermatol.
1988;119:345-350.
6.
Barth JH, Layton AM, Cunliffe WJ. Endocrine factors in pre- and postmenopausal women with hidradenitis suppurativa.
Br J Dermatol.
1996;134:1057-1059.
7.
Barth JH, Kealey T. Androgen metabolism by isolated human axillary apo-crine glands in hidradenitis suppurativa.
Br J Dermatol.
1991;125:304-308.
8.
O'Loughlin S, Woods R, Kirke PN, et al. Hidradenitis suppurativa. Glucose tolerance, clinical, microbiologic, and immunologic features and HLA frequencies in 27 patients.
Arch Dermatol.
1988;124:1043-1046.
9.
Konig A, Lehmann C, Rompel R, Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa.
Dermatology.
1999;198:261-264.
10.
Church JM, Fazio WW, Lavery IC, et al. The differential diagnosis and comorbidity of hidradenitis suppurativa and perianal Crohn's disease.
Int J Colorectal Dis.
1993;8:117-119.
11.
Attanoos RL, Appleton MA, Hughes LE, et al. Granulomatous hidradenitis suppurativa and cutaneous Crohn's disease.
Histopathology.
1993;23:111-115.
12.
Goldsmith PC, Dowd PM. Successful therapy of the follicular occlusion triad in a young woman with high dose oral antiandrogens and minocycline.
J R Soc Med.
1993;86:729-730.
13.
Sawers RS, Randall VA, Ebling FJ. Control of hidradenitis suppurativa in women using combined antiandrogen (cyproterone acetate) and oestrogen therapy.
Br J Dermatol.
1986;115:269-274.
14.
Joseph MA, Jayaseelan E, Ganapathi B, Stephen J. Hidradenitis suppurativa treated with finasteride.
J Dermatolog Treat.
2005;16:75-78.
15.
Akyol M, Ozcelik S. Non-acne dermatologic indications for systemic iso- tretinoin.
Am J Clin Dermatol.
2005;6:175-184.
16.
Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis suppurativa.
Br J Dermatol.
2006;154:726-729.
17.
Adams DR, Gordon KB, Devenyi AG, Ioffreda MD. Severe hidradenitis suppurativa treated with infliximab infusion.
Arch Dermatol.
2003;139:1540-1542.
18.
Scheinfeld N. Treatment of coincident seronegative arthritis and hidradenitis suppurativa with adalimumab.
J Am Acad Dermatol.
2006;55:163-164.
19.
Gupta AK, Ellis CN, Nickoloff BJ, et al. Oral cyclosporine in the treatment of inflammatory and noninflammatory dermatoses. A clinical and immunopathologic analysis.
Arch Dermatol.
1990;126:339-350.
20.
Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppurativa: a study of 106 cases.
Surgeon.
2005;3:23-26.
21.
Lapins J, Sartorius K, Emtestam L. Scanner-assisted carbon dioxide laser surgery: a retrospective follow-up study of patients with hidradenitis suppurativa.
J Am Acad Dermatol.
2002;47:280-285.
22.
Kagan RJ, Yakuboff KP, Warner P, Warden GD. Surgical treatment of hidradenitis suppurativa: a 10-year experience.
Surgery.
2005;138:734-740.