Best tech for Pediatrics: 2017
It’s time for Dr. Schuman’s annual year-end review of the new tech that’s changing how pediatricians care for children. Check out his recommendations!

Dr. Schuman selects and reviews products independently; manufacturers cannot pay to be listed.
It has been another outstanding year for medical technology and innovation. Without further ado, let’s get to this year’s outstanding tech to consider for your practice.
Dragon Medical One
I feel strongly that physicians should make every effort to expedite and simplify note completion. I have been using Dragon for Mac Medical for 2 years, but recently I upgraded to Dragon Medical One. This is a subscription cloud-based version of Dragon Medical that is a significant improvement upon previous versions. Dragon Medical One is 99% accurate “without initial training,” and its accuracy improves as you use it. The software speeds note completion (you can dictate 3 times faster than you can type), corrects errors easily, and utilizes voice “macros” to insert templates, phrases, and navigate from one area of the electronic health record (EHR) to another. It also uses a smartphone application (either Android or iOS) to convert your phone into a wireless microphone, complete with buttons that can be assigned to macros. If you haven’t tried voice dictation yet, consider Dragon Medical One. By doing so, you will regain valuable time and will no longer be overwhelmed by medical documentation!
PlusoptiX S16

Amblyopia, defined as poor vision caused by abnormal development of visual areas of the brain, occurs in as many as 2% to 4% of children.1 It is associated with several risk factors that include strabismus, anisometropia, astigmatism, hyperopia, and media opacity.
Related: USPSTF finalized recommendation on vision screening in kids
Since adopting the mobile plusoptiX S12C vision screener (Plusoptix Inc.; Atlanta, Georgia) in our practice more than 3 years ago, we have diagnosed countless children with ophthalmic conditions that place them at risk for amblyopia. New this year is the Vision Screener plusoptiX S16 for those practices who wish to photoscreen children in a dedicated “screening” room rather than bring a mobile screener into the exam room with the patient. The S16 features a handheld screener tethered to a base (a computer in disguise) that is connected to a monitor, mouse, and keyboard (all not included). The user interface is easy to navigate and one can print results via any network-connected printer. The S16 is priced at about $6000. Plusoptix also recently implemented a universal printer driver for Windows computers called plusoptiXconnect that facilitates sharing your findings with patients.
NEXT: The latest in vision screening tech
GoCheck Kids-iPhone version

Whereas photoscreeners like the plusoptiX S16 require practices to purchase an expensive device, GoCheck Kids (Gobiquity Inc.; Aliso Viejo, California) provides a subscription plan that utilizes a smartphone application to perform photoscreening quickly and easily. In early 2018, GoCheck Kids will release its new application that runs on a supplied iPhone 7 Plus. Using the iPhone facilitates launching the application quickly, and the large size of the phone makes it easy to perform the test. In my experience, children are very cooperative for GoCheck Kids photoscreening as they are used to having their pictures taken with their parents’ smartphones. The application works in conjunction with a web-based patient portal that retains screenings for retrieval, comparison, and review. Best of all, the subscription is less than $100 per month for unlimited screens! In my experience, reimbursement for photoscreening under code 99177 is being reimbursed in the amount of $20 to $25 dollars per screen by most insurances.
Recommended: Top 10 apps for pediatrics
Pediatric Vision Scanner (PVS)
Completing our trifecta of new vision screening devices is the Pediatric Vision Scanner (Rebion; Boston, Massachusetts) developed over a 20-year period by Dr. David Hunter, chief of Ophthalmology at Boston Children’s Hospital, Massachusetts, and cleared for marketing by the US Food and Drug Administration (FDA) in 2016. The device uses polarized laser light to test eye orientation at the retinal level to detect amblyopia in children with sensitivities and specificities much higher than those associated with photoscreeners. With existing photoscreeners, 1 in 8 children refer for ophthalmologic evaluation, and of those referred only 1 in 8 children have amblyopia or another condition requiring intervention. In contrast, with the PVS, 1 in 3 children referred will require intervention. The device is targeted for release in the second quarter of 2018. Pricing is not available as of this writing.
NEXT: Latest in point of care tech
FilmArray RP EZ
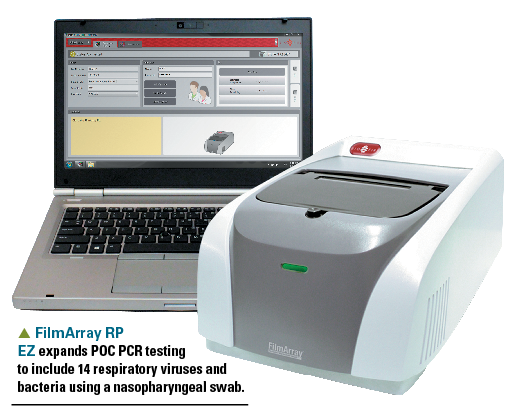
Pediatricians now have several Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88)-waived point-of care-(POC) polymerase chain reaction (PCR) systems that facilitate diagnosis of influenza A and B, strep pyogenes, and respiratory syncytial virus (RSV)-all with amazing accuracy. I’ve discussed these devices from Roche and Alere previously. FilmArray Respiratory Panel (RP) EZ from BioFire Diagnostics (Salt Lake City, Utah) expands the POC repertoire of PCR tests to include 14 respiratory viruses and bacteria. One obtains a nasopharyngeal swab, and the system processes the sample in 65 minutes. The tests include adenovirus, coronavirus, human metapneumovirus, human rhinovirus, influenza A, influenza A/H1, influenza A/H3, influenza A/H1-2009, influenza B, parainfluenza virus, RSV, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. The device is provided free of charge to practices who commit to performing 360 tests in a 1-year period. The cost per test is $130, and the reimbursement is usually 2 to 3 times the cost.
More: 10 ways to control infection
Alere Reader and BinaxNOW Influenza A and B Card

Although lateral flow POC assays for strep, RSV, and influenza A and B have been a mainstay of pediatric practices for decades, recent innovations have introduced optical readers that greatly improve their accuracy. Early in 2018, Abbott (Waltham, Massachusetts), who recently acquired Alere, will introduce a new Optical Reader that will be used in conjunction with its popular BinaxNOW Influenza A and B Card. The assay takes just 15 minutes to perform. The completed assay card is placed in the optical reader that interprets the results in seconds, eliminating operator subjectivity and improving sensitivity and specificity substantially. When purchased in quantity, each BinaxNOW Influenza A and B test costs about $16, with reimbursement typically twice that when one bills for both the A and B assays. The cost of the reader has not yet been announced.
NEXT: Latest in complete blood count tech
CLIA ’88-waived CBC device for primary care

Complete blood counts (CBCs) are routinely used by clinicians to determine if infections may warrant treatment with antibiotics. They are also useful for screening patients for anemia. Unless a pediatrician has the luxury of practicing in a clinic with an onsite lab, we must send the patient to a hospital or independent lab to have a CBC done, wasting valuable time and necessitating contacting the patient once results are available. Now for the first time ever, there is a POC CLIA ‘88-waived CBC system for primary care practices. The Sysmex XW-100 (Sysmex America Inc.; Lincolnshire, Illinois) has a small footprint (13.8 in by 7.3 in), and uses 2 liquid reagents. According to the manufacturer, the XW-100 is not for use in diagnosing or monitoring patients with primary or secondary chronic hematologic diseases/disorders, oncology patients, critically ill patients, or children aged younger than 2 years.
Next: Personalized medicine - Right drug, right patient, right time
The device will test only venous specimens (not finger-prick samples), with results available in as little as 3 minutes. The analyses include white and red blood cell count, mean corpuscular volume, platelet count, as well as neutrophil and lymphocyte count, other white blood cell counts, and neutrophil, lymphocyte, and other white blood cell percentages. The device will be available by spring of 2018. Price is not available as of this writing.
Conclusion
I hope you will consider integrating some of these innovations into your practices. For more in-depth discussion and video reviews of these technologies and others, please visit www.medgizmos.com.
REFERENCE
1. Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(11):2128-2134.e1-2.
Anger hurts your team’s performance and health, and yours too
October 25th 2024Anger in health care affects both patients and professionals with rising violence and negative health outcomes, but understanding its triggers and applying de-escalation techniques can help manage this pervasive issue.