Full case: 21-month-old with history of “skin tags” on her gluteal cleft
Read the full case presentation of a 21-month-old with a history of "skin tags" involving her gluteal cleft,noted at birth.
Case presentation
A 21-month-old girl presented with a history of "skin tags" involving her gluteal cleft, noted at birth (Figure 1A). She was otherwise healthy and developing normally. Notably, her older sister had a confirmed history of molluscum contagiosum (MC). Skin exam featured three prominent 2-4 mm soft, pedunculated papules within the superior aspect of her gluteal cleft, as well as sparsely scattered 1-2 mm papules characteristic of MC on the gluteal prominences and in mirror distribution on the perianal mucosa.
Etiology and clinical findings
A) Present case B) “Kissing” perianal distribution with an agminated lesion at the superior aspect of the gluteal cleft C) Agminated lesion within the gluteal cleft, suggesting a midline congenital anomaly D) Gluteal cleft, congenital and isolated, suggesting occult spinal dysraphism E) Gluteal cleft, condyloma-like F) Achrocordon-like and agminated lesions, unmagnified G) Achrocordon-like agminated lesion, dermatoscopic view H) Congenital agminated (sine nevus sebaceous) I) Congenital agminated (sine nevus sebaceous)
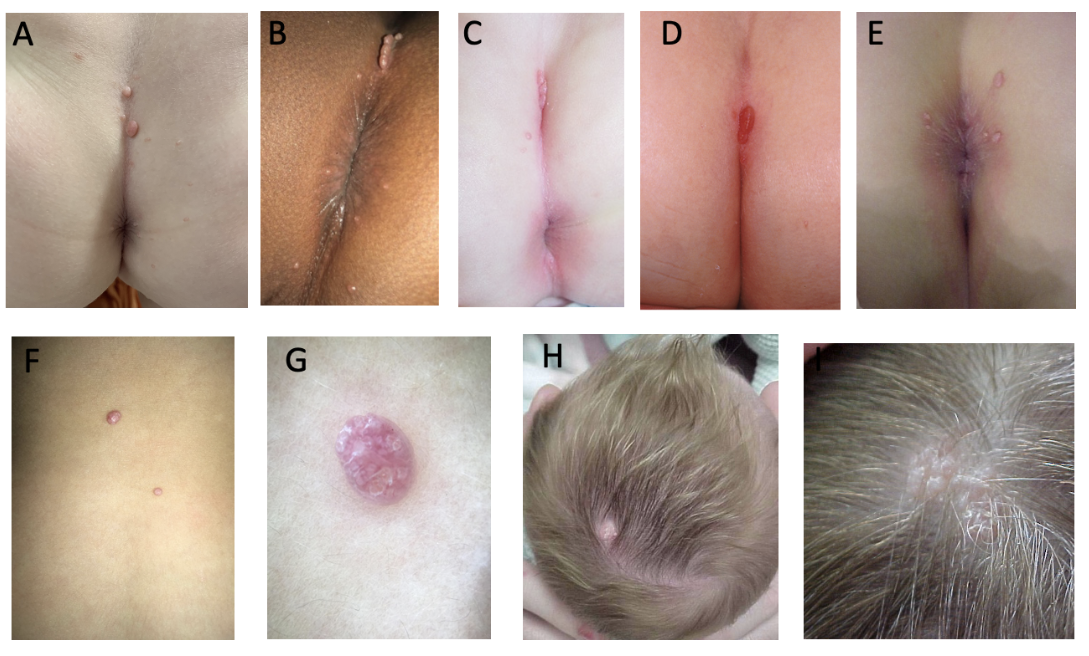
MC is a common, increasingly prevalent viral skin infection most often affecting school-aged children with an estimated point prevalence of 5% to 11% in those under age 16.1 The lesions characteristically appear as pearly, dome-shaped papules with central umbilication, typically distributed unilaterally, often on the flank and ipsilateral arm and around a skin fold in a mirror, “kissing” distribution reflecting autoinoculation (Figure 1B). The incubation period has been estimated at 2 to 8 weeks, and a history of similar lesions in a family member or close contact supports the diagnosis.
MC can also have atypical presentations that may prompt concern and unnecessary evaluation, including invasive skin biopsy. Atypical molluscum can appear as solitary, irregular, or even pedunculated papules, and involve less common sites such as the scalp and anogenital area (Figure 1C-1E). Agminated MC is another atypical variant, representing multiple clustered foci (Figure 1F-1G).
Differential diagnosis
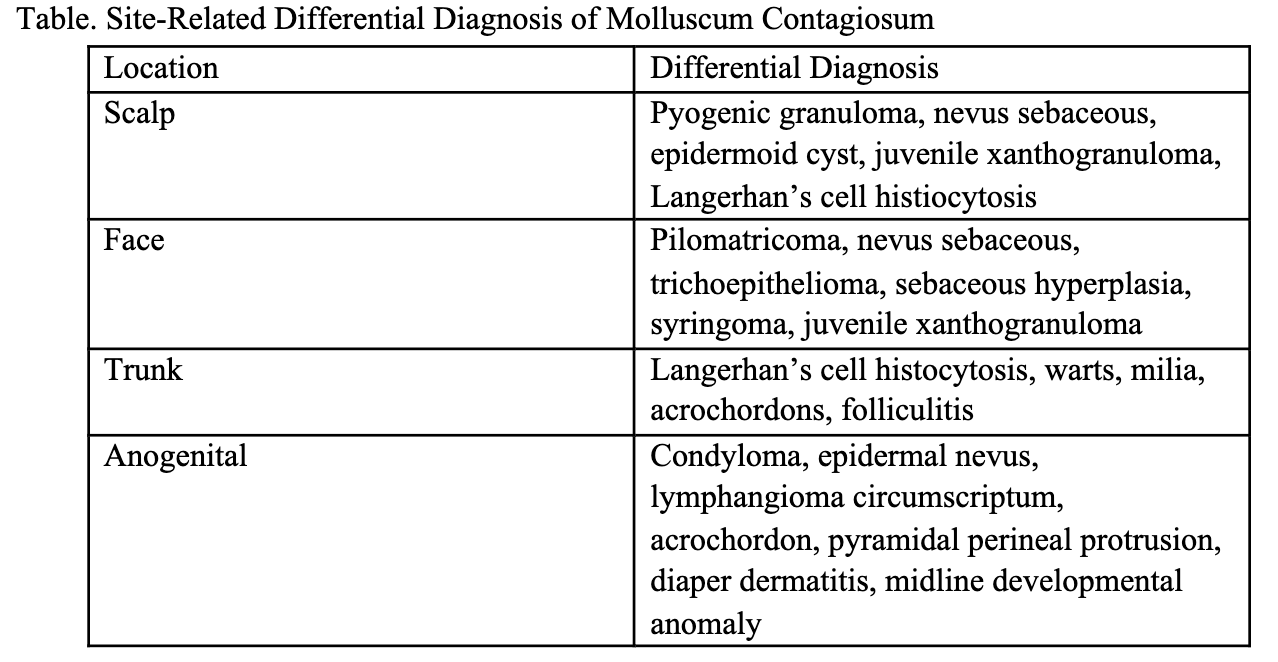
The differential diagnosis and associated extracutaneous concerns can be informed by the age at onset and distribution (Table). Congenital and neonatal-onset MC are uncommon and can have atypical, diagnostically challenging features.3,4 Congenital MC have been most often reported as isolated scalp lesions resembling nevus sebaceous, or in a halo distribution3-5 (Figure 1H-1I). Midline involvement of the gluteal cleft has been described with onset years after birth, making our case particularly unique.2 A congenital midline lumbosacral lesion can also raise suspicion of occult spinal dysraphism (Figure 1D).
Anogenital lesions in a child can suggest a sexually transmitted infection via non-innocent contact. This suspicion can be obfuscated when MC lesions are clinically indistinguishable from human papillomavirus (condyloma acuminata, Figure 1E). However, in neonatal cases, vertical transmission is likely. In all cases, it is important to maintain a high index of suspicion for abuse and confirm a reassuring social history.2
Treatment
Many over the counter (OTC) treatments are marketed for treating MC. A recent review identified nine OTC options, but the lack of comprehensively labelled ingredients and dosing information and robust clinical trials leaves healthcare providers and patients uncertain about the safest and most effective therapeutic options.6 On July 24, 2023, VP-102 (Ycanth; Verrica Pharmaceuticals) was the first medication to receive FDA approval for treatment of molluscum. This is a uniquely formulated and packaged topical cantharidin product, intended for in-office evaluation. Berdazimer gel (Novan Pharmaceuticals), a new chemical entity with anti-viral activity via nitric oxide release, is another investigational treatment currently in development and intended for daily use at home.7,8
Conclusion
In conclusion, we present a case of atypical congenital MC within the gluteal cleft and describe other clinically atypical molluscum variants to familiarize clinicians with uncommon presentations of this self-limited condition and avoid unnecessary evaluation and misdirected treatment.
Want more puzzler case studies? Click here.
References:
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130-136. doi:10.1093/fampra/cmt075
- Bakke JR, Stein SL. Molluscum Contagiosum of the Gluteal Cleft: Observations and Implications for Management in Five Children. Pediatr Dermatol. 2017;34(4):e191-e195. doi:10.1111/pde.13170
- Neri I, Liberati G, Virdi A, Patrizi A. Congenital molluscum contagiosum. Paediatr Child Health. 2017;22(5):241-242. doi:10.1093/pch/pxw009
- Connell CO, Oranje A, Van Gysel D, Silverberg NB. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25(5):553-556. doi:10.1111/j.1525-1470.2008.00730.x
- Prakashey A, Mehta H, Agrawal N, Sondagar DM. A Case of Linear Nodules on the Scalp - Dermoscopy Rules the Diagnosis. Indian J Dermatol. 2022;67(4):481. doi:10.4103/ijd.ijd_655_21
- Ong SK, Hoft I, Siegfried E. Analysis of over-the-counter products marketed to treat molluscum contagiosum. Pediatr Dermatol. 2021;38(5):1400-1403. doi:10.1111/pde.14776
- Verrica Pharmaceuticals. Verrica Pharmaceuticals Announces FDA Acceptance of Filing of Resubmitted NDA for VP-102 for the Treatment of Molluscum Contagiosum. Verrica Pharmaceuticals. Published February 27, 2023. Accessed March 1, 2025. https://verrica.com/press_release/fda-accepts-resubmitted-filing-for-vp-102-for-the-treatment-of-molluscum-contagiosum/
- Novan. Novan Submits New Drug Application to the U.S. FDA for Berdazimer Gel, 10.3% (SB206) for the Treatment of Molluscum Contagiosum. Biospace. Published January 6, 2023. Accessed March 1, 2025. https://www.biospace.com/novan-submits-new-drug-application-to-the-u-s-fda-for-berdazimer-gel-10-3-percent-sb206-for-the-treatment-of-molluscum-contagiosum
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.