Lyme disease: An update
We've learned much about diagnosing, treating, and preventing Lyme disease in the two decades since it was first described in the United States. The recent introduction of an effective vaccine has raised new hope for control, but standard protective measures remain the mainstay in avoiding disease.
Lyme disease: An update
By Alan D. Fix, MD, MS
We've learned much about diagnosing, treating, and preventingLyme disease in the two decades since it was first described in the UnitedStates. The recent introduction of an effective vaccine has raised new hopefor control, but standard protective measures remain the mainstay in avoidingdisease.
Lyme arthritis was first recognized in the United States in responseto an outbreak of arthritis among children in a small area in Connecticutin 1975.1 Those initial cases emphasize two characteristics ofLyme disease: its disproportionate incidence among children and the predominanceof arthritis among the manifestations of disseminated disease in North America--incontrast to Europe, where neurologic manifestations are more common.2
Although first described in Europe in 1883, Lyme disease was not recognizedin the US until the late 1960s.3 From the beginning of surveillanceby the Centers for Disease Control and Prevention (CDC) in 1982 through1997, over 112,000 cases were reported, and Lyme disease now accounts for95% of reported vector-borne disease.4 While increasing awarenessand reporting of Lyme disease explains a substantial portion of the trendof reported disease during the past two decades, it is not the sole explanation.The dramatic emergence of Lyme disease in the US during this period, althoughnot fully understood, appears to be associated with reforestation of areasaffected by the disease and the explosive reappearance of the deer populations.5
In the two decades since its initial recognition, much has been learnedof the pathophysiology, treatment, and prevention of Lyme disease. The recentapproval of a vaccine for persons between 15 and 70 years of age, may helpreduce the incidence of disease, but pediatricians in affected areas mustcontinue to emphasize standard methods of preventing exposure and infection.
How Lyme spreads
In North America, Lyme disease is caused by the spirochete Borrelia burgdorferisensu stricto, first identified in 1981.6 The ticks responsiblefor transmitting the disease are from the Ixodes ricinus complex. In theareas of the US where the disease is most prevalent--the northeast, mid-Atlanticand northern central regions--I scapularis is the vector. In the westernstates, I pacificus is the vector.7
Exposure to infected ticks is the crucial factor in human infection anddepends on maintaining both the organism and a population of infected ticks.In general, small rodents (particularly the white-footed mouse) supportthe population of B burgdorferi and perpetuate infection in tick populationsbecause they remain infected and apparently well for a long time, allowingthem to continue to transmit the organism to ticks. This is not the casewith white-tailed deer, which maintain the tick population but not infectionin that population.5
During their two-year life cycle, ticks pass through three stages--larva,nymph, and adult--feeding once during each stage (Figures 1 and 2). Althoughadult ticks are more likely to be infected than nymphs, the nymphs are morelikely to transmit infection because they feed in spring and early summer,when the probability of contact with human hosts is greater than in falland winter (when adult ticks transmit disease), and because they are moredifficult to detect and remove promptly than adult ticks.


Human hosts are most likely infected through tick saliva.8The process of questing (attachment and feeding of the tick) and migrationof the Borrelia organism from the tick's midgut to salivary glands and subsequenttransmission to the host requires at least 24 to 48 hours to complete. Thisfinding is consistent with animal experiments indicating a minimum of 48hours for infection to occur.9 Although infection probably occurson occasion during short periods of tick attachment to the host (less than24 hours), investigation of the risk of infection in relation to the durationof tick bite in humans has demonstrated that the risk rises substantiallyafter the tick is attached for 48 to 72 hours.10
Epidemiology and clinical manifestations
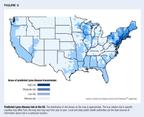
The geographic distribution of Lyme disease in the US is distinct, witheight states in the northeast, mid-Atlantic, and north-central areas (Connecticut,Rhode Island, New York, New Jersey, Delaware, Pennsylvania, Maryland, andWisconsin) accounting for over 90% of reported cases.4 Figure3 shows predicted levels of risk throughout the US. Epidemiology studieshave shown a bimodal age distribution of disease: Incidence peaks in childhoodand later adulthood, with relatively little disease reported among youngadults. The only consistent gender difference is that, among children, moreboys than girls get the disease.
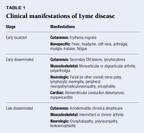
The clinical manifestations of Lyme disease--classified as early local,early disseminated, and late disseminated--are are now familiar to most(Table 1). Numerous descriptions of the spectrum of manifestations of Lymedisease exist. One must keep in mind, however, that many of these descriptionsare based on periods during which awareness and recognition of the diseasewere still developing. Thus many cases of disease were probably missed inearly stages, and many of the patients described had been referred to specialtyclinics, which resulted in obvious bias toward more advanced disease. Withimproved recognition and treatment of Lyme disease in its early stages,the proportion of patients presenting with the later manifestations hasdropped substantially. In fact, signs and symptoms of disseminated diseasewere exceedingly rare among a large cohort of subjects receiving placeboin a recent vaccine trial.11 The spectrum of disease among childrenis similar to that of adults, but with fewer long-term complications.12
The late rheumatologic manifestations of Lyme disease consist primarilyof recurrent, occasionally chronic inflammation of one or a few joints.Patients often do not respond to antibiotic therapy, and there is increasingevidence of an autoimmune basis for the condition, which shows a predilectionfor certain individuals, particularly those who are positive for human leukocyteantigens DR2 or DR4 (HLA-DR2, HLA-DR4).13 These patients alsoare more likely to have antibody to the B burgdorferi spirochete's outersurface protein A (OspA).14 Specific antigens that may be responsiblefor late rheumatologic manifestations have recently been identified.15
Making the diagnosis
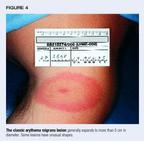
The diagnosis of Lyme disease is primarily clinical. Table 2 lists cluesto diagnosis in early disease. Although not pathognomonic, erythema migranslesions (Figure 4) occurring on persons in endemic areas do not requirelaboratory confirmation. Recent recommendations regarding laboratory testingare based on the likelihood of disease and advocate relying on testing primarilyfor patients with manifestations that suggest but do not clearly indicateLyme disease.16 Serologic tests should be used to confirm thediagnosis, not to establish it.

Inconsistent results between laboratories and even within the same laboratoryhave called into question the reliability of serologic testing and led todemands for standardization.17 Some laboratories, however, appearto have reasonably high sensitivity and specificity, especially when onedistinguishes between early and late disease.18 While the sensitivityof serologic tests is poor for early disease--and early antibiotic therapymay blunt antibody response--sensitivity in late disease is high.
False-positive enzyme immunoassay (EIA) results may arise from cross-reactivitywith other spirochetal diseases (syphilis, borrelial relapsing fever, periodontaldisease) and infectious diseases (ehrlichiosis, infectious mononucleosis,subacute bacterial endocarditis, and mumps meningitis) as well as rheumatologicillnesses (juvenile rheumatoid arthritis, rheumatoid arthritis, and systemiclupus erythematosis).5 The CDC has recommended a two-tier approachto laboratory confirmation of disease. The first tier is a sensitive EIA,and the second tier a Western blot for cases in which EIA results are eitherindeterminate or positive.19 It has been suggested that the presenceof five bands for IgG and two bands for IgM on the Western blot be usedto determine positive status.19
Assuming good sensitivity and specificity, there is no room for "screening"of asymptomatic patients for infection, even in endemic areas. The positivepredictive value of such screening would be very low, resulting in confusionover management of those screened and exposing many to unnecessary and potentiallyharmful therapy.20
Although cerebrospinal fluid (CSF) can be examined for antibodies, CSFtesting generally is not necessary to diagnose central nervous system involvementbecause almost all patients with CNS involvement have positive serologictests.21 Polymerase chain reaction testing of synovial fluidto detect B burgdorferi may prove useful in differentiating between patientswith continued joint infection and those with postinfectious inflammation,some investigators suggest.22
Treating Lyme disease
Antibiotic regimens for treating Lyme disease have generally been studiedin adults, with little attention to children, and recommended treatmentof children is similar to that for adults.12 Early local diseaseis treated with oral antibiotics. Doxycycline is the drug of choice forchildren 8 years of age or older and amoxicillin for those under 8 years(Table 3). Cefuroxime also has been approved to treat Lyme disease and isuseful for patients who cannot tolerate doxycyline or amoxicillin.
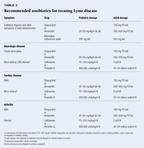
Treatment of early and late disseminated disease depends on severity.Oral therapy is recommended for less severe disease and parenteral therapyfor more severe cases. The parenteral therapy most often recommended iseither ceftriaxone or penicillin. A more detailed description of antibioticregimens for different clinical manifestations of Lyme disease has recentlybeen published.2
Duration of therapy depends on severity of illness and route of administrationbut generally varies between two and four weeks. Longer courses of antibioticsfor patients with disseminated disease remain controversial and are thesubject of ongoing study. Many regard cases of chronic arthritis unresponsiveto antibiotic therapy as an inflammatory, noninfectious condition for whichcontinued antibiotic therapy is not beneficial.
What to do about tick bites
Management of tick bites is a source of confusion. Many patients whocomplain of a tick bite have not been bitten by a deer tick and may nothave been bitten by a tick at all.10 At most, a few percent ofpatients bitten by deer ticks in endemic areas will become infected, althoughcertain patients, primarily those with tick attachment longer than 48 to72 hours, are at distinctly higher risk.
None of several placebo-controlled studies of the efficacy of prophylacticantibiotic therapy has found a statistically significant benefit.23Given the low likelihood of infection following a specific tick bite, however,the studies included too few subjects to rule out a small benefit. Indeed,the only patients who became infected in the trials were those who did notreceive antibiotic therapy. Nevertheless, antibiotics clearly would preventdisease that can be identified early and treated easily in only a coupleof patients out of every 100 receiving prophylaxis.
The general recommendation for managing tick bites is careful observationfor signs and symptoms of Lyme disease during the month following the bite,although it may be prudent to give prophylactic antibiotic therapy to patientswith prolonged tick attachment. A recent vaccine trial has demonstratedthat prospectively followed patients with appropriate awareness of the diseaseare identified early and have excellent outcomes. That study also indicatedthat probably no more than 15% have asymptomatic infection, the naturalhistory of which is unclear.11
For patients living in endemic areas, a tick bite is more significantas a marker of exposure in general than as a specific exposure to disease.The logical extrapolation of managing a specific bite with antibiotics wouldbe to "cover" the patient with antibiotic prophylaxis throughoutthe tick season, which few would consider reasonable. In addition, mostpatients with Lyme disease do not recall a tick bite, further underminingthe utility of prophylactic antibiotic treatment as a reasonable preventivemeasure.24
Another reason for caution regarding prophylactic therapy is the overlapof tick-borne diseases. Some areas where Lyme disease is endemic are alsoaffected by Rocky Mountain spotted fever (RMSF). Since many patients andphysicians cannot distinguish between the ticks that cause the two diseases,a patient may well receive prophylactic antibiotic therapy for Lyme diseasefollowing a tick bite that transmits RMSF. Prophylactic therapy for RMSFis contraindicated because it has been demonstrated to, at best, delay andconfuse the diagnosis.5
There is almost no place for serologic testing of patients who have tickbites but no symptoms of Lyme disease. Positive test results immediatelyafter the bite will either be false or indicate previous infection. Theonly rational use of testing would be to obtain acute (immediately afterthe bite) and convalescent (at least a month after the bite) specimens frompatients who remain asymptomatic in order to identify subclinical infection,and the benefit of this is unclear.
What is the prognosis?
The prognosis for patients with Lyme disease generally, and childrenspecifically, is excellent when the disease is identified and treated early.25It is less favorable for those in whom diagnosis is delayed. Even untreatedchildren, however, have experienced gradual resolution of disease, and antibiotictherapy has proved effective in children treated as long as three yearsafter onset of illness.12 Few pediatric patients have sufferedlong-term neurologic complications.26 In many patients with disseminateddisease and cranial nerve palsy, the palsy improves within 10 days of startingtreatment (or even before therapy begins). It resolves in most, but notall, patients by one month. Mortality is rare but has occurred in casesof co-infection by other, more lethal tick-borne organisms.
Much has been said about the overdiagnosis of Lyme disease in the beliefthat a substantial proportion of patients who do not respond to antibiotictherapy have symptoms that are not caused by active infection.27Such patients are more likely than those who do respond to therapy not tohave the classic manifestations of Lyme disease, not to be seropositive,and not to be suffering from Lyme disease.28 However, the spectrumof illness represented by Lyme disease as well as the manifestations ofchronic disease and what has been termed post-Lyme syndrome have been expanding.There is some suggestion that fibromyalgia in children may be a postinfectiousmanifestation of Lyme disease.12
On occasion, children have nonspecific symptoms such as headache, fatigue,and arthralgia that persist for weeks after therapy and eventually resolve.These persistent symptoms are not believed to be evidence of persistentinfection.12 It is hoped that recent successful sequencing ofthe genome will allow greater advances in understanding pathogenesis andlead to advances in treatment and prevention.29
Preventive measures
Avoiding exposure to ticks--by minimizing exposure to areas they infest--andtick attachment remain the primary means of preventing infection, even withthe availability of vaccine. Table 4 lists recommended preventive strategies.
The insect repellent deet (N,N-diethyl-m-toluamide) is very effectivebut must be used cautiously on children and not applied to the face or hands.Warn caregivers to apply low concentrations (10% or less) and to wash theskin with soap and water once the child comes indoors.
The skin should be examined carefully for attached ticks at least oncea day, bearing in mind the small size of nymphal ticks, the most commonsource of infection. Frequent sites of attachment include the groin, poplitealfossae, gluteal folds, axillary folds, and ear creases.30
It is crucial for physicians and patients to be aware that an episodeof Lyme disease does not confer immunity. There are clear reports of reinfection.31
The Lyme vaccine
Two human vaccines against Lyme disease have been developed and evaluated.11,32Both are based on recombinant OspA antigen, which is expressed in the tickbut not in the human host until late in disease. The vaccine presumablyworks by actively eliciting antibody in humans that kills the Borrelia organismin the tick's midgut during feeding, before it can migrate to the tick'ssalivary glands and be transmitted to the host.
One of the vaccines, LYMErix (SmithKline Beecham), was recently approvedby the Food and Drug Administration for administration to patients between15 and 70 years of age, and the CDC has published recommendations for itsuse (see box, "The CDC recommendations for using Lyme disease vaccine").33Some physicians are concerned about long-term safety, particularly amongthose who may be predisposed to the late, presumably autoimmune-mediated,manifestations of Lyme disease. Thus far, however, there has not been evidenceto support this concern. Trials are under way to evaluate the vaccine'sefficacy and safety in children under 15 years of age.

LYMErix is given in three doses of 30 mg each at 0, 1, and 12 months.Depending on how one defines infection and disease, the vaccine has beenshown to be between 68% and 76% effective in preventing infection duringthe year following the initial three doses. It is not yet clear whetherand how often booster doses will be necessary, but the current theory ofhow the vaccine works would necessitate maintaining relatively high antibodylevels.
Although the vaccine is expected to have a positive impact in areas whereLyme disease is endemic, several cautionary notes are in order. Since thevaccine will likely prevent only about two thirds to three quarters of Borreliainfections, physicians must continue to consider Lyme disease in the differentialdiagnosis of illness with a compatible clinical presentation. EIA testswill not be helpful in evaluating patients who have received vaccine becausetest results are expected to be positive following vaccination. Westernimmunoblot testing must be used in this situation.
Perhaps most important, physicians must advise patients that the vaccinedoes not protect everyone and that even vaccinated persons must assume theyare still at risk of infection. Therefore, they must not abandon the standardpreventive measures outlined above.
THE AUTHOR is Assistant Professor in the Department of Epidemiologyand Preventive Medicine at the University of Maryland School of Medicine,Baltimore.
REFERENCES
1. Steere AC, Malawista SE, Snydman DR, et al: Lyme arthritis: An epidemicof oligoarticular arthritis in children and adults in three Connecticutcommunities. Arthritis Rheum 1977;20:7
2. Nadelman RB, Wormser GP: Lyme borreliosis. Lancet 1998;352:557
3. Burgdorfer W: Discovery of Borrelia bergdorferi, in Coyle PK (ed):Lyme Disease. St. Louis, Mosby-Year Book, 1993, pp 37
4. Dennis DT: Epidemiology, ecology, and prevention of Lyme disease,in Rahn D, Evans J (eds): Lyme Disease. Philadelphia, American College ofPhysicians, 1998, pp 734
5. Walker DH: Tick-transmitted infectious diseases in the United States.Annu Rev Public Health 1998;19:237
6. Burgdorfer W, Barbour AG, Hayes SF, et al: Lyme disease--A tick-bornespirochetosis? Science 1982; 216:1317
7. Piesman J, Gray JS: Lyme disease/Lyme borreliosis, in Sonenshine DE,Mather TN (eds): Ecological Dynamics of Tick-Borne Zoonoses. New York, OxfordUniversity Press, 1994, p 327
8. Zung JL, Lewengrub S, Rudzinska M, et al: Fine structural evidencefor the penetration of the Lyme disease spirochete Borrelia burgdorferithrough the gut and salivary tissues of Ixodes dammini. Can J Zool 1989;67:1737
9. Piesman J, Maupin GO, Campos EG, et al: Duration of adult female Ixodesdammini attachment and of Borrelia burgdorferi, with description of a needleisolation method. J Infect Dis 1991;163:895
10. Sood SK, Salzman MB, Johnson BJ, et al: Duration of tick attachmentas a predictor of the risk of Lyme disease in an area in which Lyme diseaseis endemic. J Infect Dis 1997;175:996
11. Steere AC, Sikand VK, Meurice F, et al: Vaccination against Lymedisease with recombinant Borrelia burgdorferi outer-surface lipoproteinA with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med 1998;339:209
12. Shapiro ED: Lyme disease in children, in Rahn D, Evans J (eds): LymeDisease. Philadelphia, American College of Physicians, 1998, pp 123135
13. Steere AC, Dwyer E, Winchester R: Association of chronic Lyme arthritiswith HLA-DR4 and HLA-DR2 alleles. N Engl J Med 1990;323:219
14. Kalish RA, Leong JM, Steere AC: Association of treatment-resistantchronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA andOspB of Borrelia burgdorferi. Infect Immun 1993;61:277
15. Gross DM, Forsthuber T, Tary-Lehmann M, et al: Identification ofLFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis.Science 1998; 281:703
16. Tugwell P, Dennis DT, Weinstein A, et al: Laboratory evaluation inthe diagnosis of Lyme disease. Ann Intern Med 1997;127:1109
17. Bakken LL, Callister SM, Wand PJ, et al: Interlaboratory comparisonof test results for detection of Lyme disease by 516 participants in theWisconsin State Laboratory of Hygiene/College of American Pathologists ProficiencyTesting Program. J Clin Microbiol 1997; 35:537
18. Craven RB, Quan TJ, Bailey RE, et al: Improved serodiagnostic testingfor Lyme disease: Results of a multicenter serologic evaluation. EmergingInfectious Diseases 1996;2:136
19. Centers for Disease Control and Prevention: Recommendations for testperformance and interpretation from the Second National Conference on SerologicDiagnosis of Lyme Disease. MMWR 1995;44:590
20. Seltzer EG, Shapiro ED: Misdiagnosis of Lyme disease: When not toorder serologic tests. Pediatr Infect Dis J 1996;15:762
21. American College of Physicians: Guidelines for laboratory evaluationin the diagnosis of Lyme disease. Ann Intern Med 1997;127:1106
22. Nocton JJ, Dressler F, Rutledge BJ, et al: Detection of Borreliaburgdorferi DNA by polymerase chain reaction in synovial fluid from patientswith Lyme arthritis. N Engl J Med 1994;330:229
23. Warshafsky S, Nowakowski J, Nadelman RB, et al: Efficacy of antibioticprophylaxis for prevention of Lyme disease. J Gen Intern Med 1996;11:329
24. Dennis DT, Meltzer MI: Antibiotic prophylaxis after tick bites. Lancet1997;350:1191
25. Gerber MA, Zemel LS, Shapiro ED: Lyme arthritis in children: Clinicalepidemiology and long-term outcomes. Pediatrics 1998;102:905
26. Adams WV, Rose CD, Eppes SC, et al: Cognitive effects of Lyme diseasein children: A four-year followup study. J Rheumatol 1999;26:1190
27. Steere AC, Taylor E, McHugh GL, et al: The overdiagnosis of Lymedisease. JAMA 1993;269:1812
28. Fawcett PT, Rose CD, Gibney KM, et al: Correlations of seroreactivityin response to antibiotics in pediatric Lyme borreliosis. Clin Diagn LabImmunol 1997;4:85
29. Fraser CM, Casjens S, Huang WM, et al: Genomic sequence of a Lymedisease spirochaete, Borrelia burgdorferi. Nature 1997;390:580
30. Rahn DW: Natural history of Lyme disease, in Rahn D, Evans J (eds):Lyme Disease. Philadelphia, American College of Physicians, 1998, pp 3548
31. Nowakowski J, Schwartz I, Nadelman RB, et al: Culture-confirmed infectionand reinfection with Borrelia burgdorferi. Ann Intern Med 1997;127:130
32. Sigal LH, Zahradnik JM, Lavin P, et al: A vaccine consisting of recombinantBorrelia burgdorferi outer-surface protein A to prevent Lyme disease. RecombinantOuter-Surface Protein A Lyme Disease Vaccine Study Consortium. N Engl JMed 1998;339:216
33. Centers for Disease Control and Prevention: Recommendations for theuse of Lyme disease vaccine. Recommendations of the Advisory Committee onImmunization Practices (ACIP). MMWR 1999;48(RR-7):1, 21
Alan Fix. Lyme disease: An update. Contemporary Pediatrics 1999;9:77.