Nasal allergy: More than sneezing and a runny nose
For many children, allergic rhinitis is not just a seasonal misery but a persistent condition that can adversely affect overall health. Recognition and effective treatment are the keys to minimizing complications.
Nasal allergy: More than sneezing and a runny nose
By William R. Solomon, MD
For many children, allergic rhinitis is not just a seasonal misery but a persistent condition that can adversely affect overall health. Recognition and effective treatment are the keys to minimizing complications.
Physician views of nasal allergy are dominated by its high prevalence, often dramatic seasonal nature, and largely benign prognosis. In North America, rates of nasal allergy (allergic rhinitis) approach 8% to 12% in the general population and more than 20% in college student groups.1 Recognizable seasonal allergy appears most often between 6 and 10 years of age, but is not unknown in toddlers, who are experiencing their second annual pollen exposure. Most children with nasal allergies simply accept their symptoms or find tolerable comfort using proprietary antihistaminic medications.
An important minority of affected children, however, suffer perennial discomfort, reflecting exposure to agents such as mites or pets in the home or school. Coryza and itching often are minor complaints, with morbidity and complications arising primarily from nasal blockage. In many of these children, seasonal coryza with itching and eye symptoms, usually caused by pollen allergy ("hay fever"), is superimposed on persistent nasal obstruction. Seasonal nasal allergy by itself can cause edema of the paranasal sinuses and eustachian tube dysfunction with middle ear effusions, both of which facilitate secondary bacterial infection. These problems are far more troublesome when compounded by the persistent nasal blockage of perennial disease.
Increasing recognition of the potential impact of nasal allergy on children's health has led to greater emphasis on cost-effective management by primary care physicians. Pediatricians need to know how to identify allergic rhinitis and what treatments are effective as well as when subspecialty consultation is indicated.
Adverse effects of nasal allergy
A history of "stuffy nose" since birth is common in children with perennial allergies, especially when food allergy contributes to nasal obstruction but also without it. In addition, distraught parents describe recurrent catarrhal otitis media, bronchitis, and nasal suppuration, with or without documented sinus disease. A complex of nasal allergy, chronic nasal suppuration, and adenoid enlargement often becomes evident as investigation proceeds. Although the exact role of purulent secretions in promoting asthma is not fully defined, few clinicians would deny the association.
Other adverse health effects of nasal blockage are equally compelling. Established associations exist between persistent (obligatory) mouth breathing and patterns of facial maldevelopment including overbite, marked palatal arching, and malar flattening. Infraorbital darkening and edema further reflect nasal congestion and, with a gaping mouth, heighten a picture of "precocious dissipation." This complex has been termed "the allergic facies" but is the common result of any prolonged nasal obstructive state.2
Such children are often just as listless and drowsy as their appearance suggests. Sleep studies confirm that nasal blockage, even without obstructive apnea, prompts recurrent microawakenings and limits the depth of sleep, especially the REM phase. Successful treatment of the obstruction often results in dramatically heightened affect, alertness, and activity.

Identifying allergic rhinitis
"The allergic nose," above, describes the physiologic process that produces the tissue changes of allergic rhinitis. Convincing evidence for nasal allergy develops when symptoms compatible with the diagnosis occur in conjunction with defined allergen exposure and relevant, IgEbased sensitivity. Unfortunately, even these requirements may be difficult to establish when complaints are continuous or exposures inapparent. Yet nasal symptoms that occur predictably in the presence of house dust, pets, certain foods (rarely), or seasonal exposures strongly suggest allergy as one determinant. Errors of inference easily result when implicated exposures are also potent nonspecific irritants that cause symptoms in persons other than those with allergies. Similarly, symptoms occurring during familiar March-April and September-October peaks of viral illness are readily mistaken for symptoms of seasonal pollen allergy. Table 1 lists factors in the patient's history that favor a diagnosis of nasal allergy.

Physical evaluation aids in diagnosis by excluding entities such as occult infection, polyps, foreign bodies, and adenoid enlargement. The physician may seek in vain, however, for the legendary pale, bluish mucosa of nasal allergy, which is often obscured by the effects of nose rubbing, infection, or exposure to irritants.
In many youngsters with nasal blockage, purulent rhinorrhea, and middle ear disease, evaluation fails to implicate significant allergic factors and other conditions must be considered. Assessment of such children by a pediatric otolaryngologist can be highly cost-effective if an anatomic factor that impairs sinus drainage can be identified--and subsequently corrected.
Questions of chronic sinusitis are best resolved by a dedicated CT scan with osteomeatal (principal drainage pathway) views. This examination, using 3-mm "cuts," costs little more than plain maxillofacial films and provides a "gold standard" for visualizing intracavitary and intranasal anatomy. Additional studies for children with recurrent infection may include a sweat test and assay of quantitative immunoglobulins and IgG subclasses, if exclusion of other entities leaves humoral immunodeficiency as a possible determinant.
Specific antibody responses to protein (tetanus) or carbohydrate (pneumococcal capsular type) immunogens are readily measured and significant for evaluating humoral immune competence. Antipneumococcal antibody titres, measured both before and after administering Pneumovax, provide a useful indicator of response to polysaccharide antigens involving especially IgG2 and IgG4.
"Allergy testing," as an indicator of allergen-specific IgE in skin or serum, offers diagnostic insight on two levels. Strong positive response to one or more environmental agents implies that the patient is "atopic," hence potentially at risk for conditions such as nasal allergy, allergic asthma, and atopic eczema. Specific IgE positivity implies a predisposition to allergy symptoms when the patient encounters corresponding agents such as pollens and dust mites. Useful inferences about cause become possible when a correlation exists between specific IgE and circumstances that have provoked symptoms.
Skin or blood tests alone, without suggestive historical detail, however, cannot establish a cause for symptoms with practical certainty. Although high-titered positive tests often occur when allergens do provoke clinical symptoms, exceptions--positive tests but no symptoms--are too numerous to ignore. By contrast, negative allergen-specific test findings, when the skin wheals normally with histamine or a histamine liberator such as codeine, casts significant doubt on IgE-mediated sensitivity as a determinant of clinical symptoms.
In experienced hands, direct skin tests remain the preferred method of evaluating the presence of allergen-specific IgE. In vitro studies (RAST, FAST, MAST, ELISA) often are more costly and difficult to perform with consistent accuracy. In vitro testing may be the only option, however, when materials or expertise for skin testing are lacking or when skin testing is contraindicated by such factors as persistently nonreactive positive control responses, whole-body rash, or prior skin testinduced anaphylaxis. Large, highly experienced laboratories offer the best chance of obtaining reliable results.
Other types of tests have limited value. Assays of total serum IgE are particularly unrewarding. Profound nasal secretion eosinophilia is strongly associated with IgEmediated allergy but may occur in other nasal conditions or, occasionally, be absent, especially with active bacterial infection.
Significant allergens in allergic rhinitis
The most familiar offenders in nasal allergy are airborne plant pollens and emanations of fungi, arthropods (mites and insects), and furry mammals (especially pets). It is noteworthy that all of these have biological sources, and the responsible molecular allergens are small to mid-sized proteins. Furthermore, allergenic particles generally range in size from 5 to 70 mm, facilitating aerial transport and favoring deposition in the upper airway. Many commercial materials derived from natural sources have caused local allergic outbreaks by this means, notably latex, dusts from soy and castor beans, bacterial enzymes (such as the enzymes from Serratia marcescens that are used in cleaning products), and grain elevator aerosols.
Although airborne agents are the primary allergens, especially in childhood, food allergy cannot be ignored as a cause of nasal symptoms. Cow's milk seems most problematic, causing symptoms that often begin shortly after birth. Less often, eggs, peanuts, corn, and wheat cause symptoms when reintroduced to the diet individually after a period of withdrawal. Maternal dietary components may, rarely, precipitate allergic symptoms in exclusively breastfed infants. Food allergy that produces only nasal symptoms is quite uncommon, however, and seldom continues beyond the preschool years. Table 2 lists allergens that contribute to allergic rhinitis.

Dust mites
Of all the many familiar arthropods to which skin testing demonstrates sensitivity, dust mite emanations have by far the greatest clinical allergic impact. A few varieties of mites use human squames (shed epidermal cells) as food and avidly colonize homes. Populations, which include adults and larval forms, usually are highest in bedding, upholstery and floor coverings. Dominant indoor varieties vary among climatic regions, but, as a group, dust mites thrive wherever people live.
Lacking means to conserve water, dust mites must depend on ambient moisture and rapidly die in dry surroundings. Strategies that lower indoor humidity can reduce mite numbers, which often vary seasonally, with summer-fall peaks and winter-spring troughs. Allergens in dust can remain potent for many weeks, however.
The principal vector of mite allergens is the fecal pellets, which are 20 mm in diameter and enclosed in a membranous envelope. Particles of this size (comparable to a ragweed pollen grain) become airborne easily with domestic activity but also fall out rapidly when the disturbance ends. While their time in the air is relatively short, factors such as furnace fans greatly prolong suspension.
Reducing indoor relative humidity is critical to controlling the population of dust mites and thereby minimizing human exposure. Encasing bedding and upholstery in sheet plastic or microfiber fabrics provides a cost-effective, complementary approach. Newer, finely felted fabrics appear to give good protection without the poorly tolerated "feel" of plastic. Washing bedding in very hot water (155° or above) for at least 10 minutes usually kills dust mites if the temperature can be achieved consistently (which is more likely in commercial laundries than home washing machines).
Since vacuuming alone does little to remove mites from carpeting, applications of benzyl benzoate powder or foam to carpets may be used to kill the acarids. Treatment must be repeated periodically, however, and human exposure to the chemical must be minimized.
Animal allergens
Respiratory sensitivity to animal allergens poses some of the thorniest management problems for physicians. Given a suitably prolonged exposure, human beings probably can develop sensitivity and symptoms to virtually any mammalian species. Among children, however, house pets, especially cats, are the principal offenders.
Animal allergens tend to be potent and distinctive to each mammalian family (and often species), with multiple sources on the animal's body, including saliva, urine, lacrimal secretions, and epidermal scales. Aerosol particles that carry allergen, especially cat allergen, are often a few mm in diameter, or even submicronic. Unlike dust mite fecal pellets, they can remain airborne almost indefinitely once they are released. Their small size also promotes diffusion to surfaces such as walls, hair, and clothing, accounting in part for the high levels of cat and dog allergens found in some public places, including schools. Animals brought to school for visits or residing there as classroom pets (often small rodents or rabbits) can severely compromise the allergic child during prolonged exposure.
Rarely, clothing made from animal hair serves as an important allergen vector. Garments containing horse and camel hair as well as mohair and angora deserve special mention. Fur trim and linings (especially rabbit fur) can likewise cause problems. Also, toys originating abroad may contain dog and cat fur or other (unlabeled) natural materials.
Total avoidance of an offending animal often is the price of acceptable health for sensitive patients. Bonding with pets by household members, including the allergic person, commonly precludes this solution, however. Keeping the animal outdoors consistently may help, although climate or the danger of predators limits that option in many areas.
Effective means of reducing allergen release by pets have not yet emerged. Some hints of benefit from washing cats weekly are reported, but the issue remains undecided, and possible beneficial effects of bathing dogs are purely speculative. Developing animals that produce or release low allergen levels is a promising idea, but present knowledge does not support any existing dog or cat breed as preferable for allergic persons.
Pollens
Annually recurring symptoms of repetitive sneezing, voluminous rhinorrhea, and intense pruritus have made pollinosis the best-recognized allergy problem. Of the roughly 10% of flowering plants that shed airborne pollen, many produce some degree of human sensitization, including numerous trees in temperate regions, grasses, and a few groups of broad-leafed, nonwoody species, such as the ragweeds.
Allergenic pollens are released profusely during species-specific flowering seasons. Trees usually bloom earliest and show the greatest annual variation in dates of pollen release and total output. Depending on location, grass pollen emissions may span the entire growing season or just six to eight weeks in early summer. The late July to late September period of ragweed pollinosis is probably the most predictable pollen season in its timing.
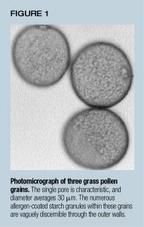
At any season, pollen release depends on the weather and is strongly favored by warm, dry, breezy conditions. Determinants of allergen exposure are complex, however, making generalizations risky. A substantial portion of pollen allergens, for example, are carried by aerosol particle fractions far smaller than intact grains. In the case of grass pollen, osmotic lysis can release allergen-bearing subcellular starch granules (see figure 1). Well-documented outbreaks of grass allergen-induced asthma and rhinitis during thunderstorms seem to reflect this effect.4 For other pollens, however, the factors favoring allergen carriage by small particles are unknown.
These uncertainties do not diminish the value, for sensitive persons, of avoiding obvious pollen sources when feasible. Focusing on indoor activities during pollen seasons is helpful, especially if buildings are closed and air conditioned for comfort. Even without central air conditioning, a closed bedroom with a window unit that is operated only when the room is occupied can reduce daily pollen exposure. Houseplants are virtually never clinically important sources of pollen (or fungus) allergens.
Outdoor pollen levels tend to be highest from mid-morning to late afternoon, although their impact on symptoms probably is far more extended. In fact, as in much nasal allergy, coryza caused by pollinosis typically is worst when a person arises in the morning, even though pollen levels may be at their diurnal lows.
Trees and shrubs that produce wind-borne pollen and resulting hay fever have small, drab, clustered flowers that totally lack attractants (large size, bright colors, sweet scents) for insect pollinators. Ornamental floral plants, by contrast, generally play no role in human allergy and are preferable choices for planting where allergy is a concern. Detailed calendars describing pollen exposure at different times of the year are available by state and region.5,6
Fungi
As organisms quite different from both plants and animals, fungi present distinctive allergens as well as singular problems of detection and control. Because most airborne fungus particles arise from microscopic growth in nature, direct air sampling is needed to document sources and estimate the risks of exposure. In such studies, fungi usually constitute the most numerous biogenic particles in air with significant attributable clinical effects on allergic individuals.
Efforts to limit inhalant exposure to fungi probably are best focused outdoors where these organisms are prominent saprophytes and parasites. Avoiding infected crops and refraining from disturbing plant compost is paramount. Even familiar activities such as cutting grass or field crops, raking leaves, or merely walking through knee- high vegetation can increase exposure dramatically. Airborne fungus levels usually are highest in grain-growing areas where harvesting and hot, breezy weather increase prevalence during the day. At night and during rain, a different population of fungi, about which much less is known, dominate the air. Many of the particles from these latter fungi are splash-dispersed, as with yeasts, or consist of spores actively released by fleshy fungi.
Although outdoor exposures account for much of the clinical impact of fungi, indoor sources also can be critical. Given adequate moisture, even nutritionally barren, man-made surfaces will support some growth. Organic materials such as wood, cotton, jute, and straw can, if moist, harbor large numbers of microbes. Besides promoting the growth of fungi (as well as dust mites and roaches), indoor humidity often introduces additional fungi into the air if it arises from contaminated sources. Poorly maintained cool-mist vaporizers are principal offenders and require daily scouring when in regular use.7 Evaporative units pose less risk but are awkward to maintain. Commercial additives offer uncertain, or at least unproven, protection.
Maintaining adequate indoor moisture (not exceeding 40% relative humidity) is a legitimate concern for patients with purulent respiratory infection, cystic fibrosis, or eczema, especially in dry winter periods. Excessive home dampness, however, has been associated independently with childhood respiratory ill health in both allergic and nonallergic groups.8 The idea that volatile agents from organisms such as fungi that are tissue irritants may cause symptoms remains intriguing but unproven.
A few more allergens
Since a host of protein-associated airborne agents can elicit specific IgE responses, the list of reported inhalant allergens is extensive. Cockroach emanations are an acknowledged factor in childhood asthma and rhinitis, usually in urban areas. Rarely, local "emergences" of other insects, such as mayflies, are implicated. Seed dusts harboring potent allergens sometimes are found in beanbag toys and cushions.
Respiratory allergy caused by latex sensitivity can occur where daily health care mandates regular use of rubber gloves, especially powdered gloves. The issue of whether ambient levels of airborne latex, as from vehicle tire wear, causes symptoms remains controversial.
Managing nasal allergy
Although available treatment agents and tactics change with time, the value of avoiding allergens when feasible, using suppressive medications, and administering allergen-specific immunotherapy remains established.
Avoiding allergens. Measures such as those mentioned previously to avoid specific "indoor" sources of allergens (dust mites, pets) provide benefit--often at low cost. Air-conditioned interiors offer refuge from outdoor pollens and fungi. Selective dehumidification is also valuable, as explained above. Domestic air cleaners, however, afford only limited benefit, even with indoor allergens. Evaluation by an allergist can provide both a firm basis and a practical strategy for implementing appropriate individualized avoidance measures. Table 3 lists objectives of referral to an allergist.

Suppressive medication remains the most widely chosen means of controlling nasal allergy symptoms because of its frequent effectiveness and relative convenience. Affected persons typically rely on oral H1 blocking antihistaminics, taken as needed, and, for many, these suffice.9 Older ("sedating") and newer ("non-sedating") agents offer similar efficacy, differing primarily in CNS penetration. Finding the best agent for a specific patient, however, often requires a trial-and-error search. The Physician's Desk Reference lists the varied available agents under "antihistamines and combinations." Some preparations of special value in young children who resist taking pills are noted in Table 4.

The patently exhausting effect of allergic coryza coupled with poor sleep leaves little room for drugs causing further daytime sedation. Of the available agents, loratadine (Claritin) and fexofenadine (Allegra) are essentially nonsedating while cetirizine (Zyrtec) rarely causes drowsiness. At least half of persons taking other agents report drowsiness. Adding an older, sedating agent at bedtime, however, may provide incremental benefit, especially for rhinorrhea, without causing unacceptable early morning drowsiness. Numerous trials have confirmed that H1 blockers have a negligible effect on nasal blockage, for which a decongestant (usually pseudoephedrine or phenylpropanolamine) may be added to the drug regimen. H2 blockers have no confirmed role in rhinitis therapy.
Tolerance for oral decongestants varies remarkably among patients, and potential side effects on intraocular pressure, cardiovascular responses, bowel and bladder tone, and seizure thresholds deserve attention. Habitual use of topical decongestants leading to "rebound" blockage is a special concern in adolescents. Five to seven days of indicated treatment carries little or no risk, however, and the "safe" period often can be extended by using topical agents every other day.
Several other types of topical medication--including azelastine (Astelin), cromolyn sodium, and corticosteroids--are useful in nasal allergy. In addition to these drugs, saline sprays, gels, and drops help loosen thick secretions and reduce mucosal dryness. Generic saline preparations should offer price advantages, but often burn briefly after administration, perhaps because of the preservatives they contain or their tonicity.
When oral antihistamines do not alleviate symptoms, topical azelastine is sometimes useful as primary or complementary therapy. The preparation's bitter taste can limit its use, however, and ready nasal absorption occasionally allows sedative side effects to emerge.
Topical cromolyn sodium, an agent of legendary safety that is available without prescription, has value in managing uncomplicated nasal allergy. The drug is especially useful to prevent symptoms before predictable and, preferably, finite allergen exposures. Where the allergic challenge is prolonged, it must be given at least three times a day--four times is better--for maximum benefit. A one- to two-minute interval between sprays may prevent the dose from draining rapidly into the throat or nostril. Deposits of dried drug sometimes seen around the nares are seldom more than a curiosity.
Topical corticosteroids have strong antiallergic properties when used intranasally and are often combined in regimens with oral antihistamines. Most intranasal steroid preparations can now be given effectively once or twice a day. An array of agents of varying potencies are available, some hand pumped, some using a propellant, some scented, some not. In children with intractable nasal congestion a brief course of a local decongestant or oral corticosteroid or even surgery may be needed to facilitate aerosol penetration.
Side effects of intranasal steroids are few and confined largely to local bleeding, which may be subtle or profuse. Especially in children, nose picking increases the risks of bleeding, and as many as 7% may experience bleeding during regular treatment. Reducing the dosage or adding nasal saline often can allow continued, safe administration without epistaxis.
Since the nasal mucous membrane is an avid absorptive surface, concerns about systemic side effects, specifically growth suppression, are relevant. More than an decade of experience, however, has shown that systemic complications are virtually absent when approved dosing guidelines are followed. Nevertheless, topical steroids probably should not be used where oral antihistaminics suffice. Periodic follow-up is essential, both to assess treatment needs and to limit possible complications.
Allergen-specific immunotherapy is a proven approach to managing nasal allergy and should be considered when avoidance measures and symptom-suppressant drugs options fail. While the mechanism(s) responsible for the efficacy of immunotherapy are not fully understood, induced changes in allergen-specific T-lymphocyte function seem especially important.
In appropriately selected patients, significant symptom improvement has accompanied treatment with extracts of dust mites, ragweed, grass and several tree pollens, some principal outdoor fungi, and cat hair or pelt. Current guidelines suggest at least three to five years of treatment when early phase assessments indicate symptomatic benefit. Twelve to 15 months may elapse initially, however, before convincing subjective improvement occurs.
Immunotherapy must be given by personnel experienced in its use at a medical facility adequately staffed and equipped to deal with possible adverse events. Minimal resources should include two trained care providers; injectable epinephrine, diphenydramine and corticosteroids; intravenous fluids; a pressor agent; and a respiratory assist device such as an Ambu bag. At least 1% to 2% of patients experience a systemic reaction at some time during treatment. Most reactions consist of briefly increased respiratory symptoms, but a full anaphylactic syndrome demanding prompt treatment and, occasionally, recurring after three to eight hours, may appear. Principal predictors of such adverse effects include prior adverse reactions, intense skin sensitivity to an injected allergen, concurrent heavy allergen exposure, and any incidental state of hypermetabolism, which can increase perfusion of an injection site.
Tailor the treatment to the child
As awareness of the morbidity and potential complications of childhood nasal allergy has grown in recent years so has emphasis on treatment by primary care physicians. It is important to recognize that the population of children who suffer from allergic rhinitis is far from homogeneous and that treatment needs vary widely. Using a "stepped," or incremental, approach should help both avoid unnecessary treatment and prevent morbidity. When simpler approaches fail, however, an allergist often can provide cost-effective help in improving quality of life for the affected child.
THE AUTHOR is Professor of Internal Medicine, Division of Allergy and Clinical Immunology, The University of Michigan Health System, Ann Arbor.
REFERENCES
1. Smith JM: Epidemiology, in Mygind N, Naclerio R (eds): Allergic and Non-Allergic Rhinitis. Copenhagen, Munksgaard, 1993
2. Marks MB: Significance of discoloration in the lower orbitopalpebral grooves in allergic children (allergic shiners). Ann Allergy 1963;21:26
3. Wachs M, Proud D, Lichtenstein LM: Observations on the pathogenesis of nasal priming. J Allergy Clin Immunol 1989;84:492
4. Suphioglu C: Thunderstorm asthma due to grass pollen. Int Arch Allergy Immunol 1998;116:253
5. Chang WWY: Pollen survey of the United States, in Grammer LC, Greenberger PA (eds): Allergic Diseases: Diagnosis and Management, ed 5. Philadelphia, Lippincott- Raven, 1997
6. Solomon VIR, Platts-Mills TAB: Aerobiology and inhalant allergens, in Middleton E Jr, Reed CE, Ellis EF, et al (eds): Allergy: Principles and Practice, ed 5. St. Louis, CV Mosby, 1998
7. Solomon WR: Fungus aerosols arising from cool-mist vaporizers. J Allergy Clin Immunol 1974;54:222
8. Platt SD, Martin CJ, Hunt SM, et al: Damp housing, mould growth and symptomatic health state. BMJ 1989;293:1673
9. Simons FER: H1-receptor antagonists, comparable tolerability and safety. Drug Safety 1994;10:350
William Solomon. Nasal allergy: More than sneezing and a runny nose. Contemporary Pediatrics 1999;8:115.