Pulse oximetry: The fifth vital sign
It is easy to take for granted some of the technologies we use every day. The pulse oximeter was invented 40 years ago and has become such a routine part of medical practice that oximetry measurements have often been referred to as the “fifth vital sign.”
It is easy to take for granted some of the technologies we use every day. The pulse oximeter was invented 40 years ago and has become such a routine part of medical practice that oximetry measurements have often been referred to as the “fifth vital sign.” This article takes a look at the brief history of pulse oximetry, describes how the technology works, and details the nuances of using pulse oximetry in everyday pediatric practice.
From humble beginnings
Modern day pulse oximetry had its beginnings in avionics research. In the early days of high altitude flight, it was important to study the effects of cabin pressure on the oxygenation of blood in the circulatory system of pilots. In 1935, Karl Matthes, a German scientist, introduced the first ear oxygen saturation meter using 2 photo sensors and lights initially in the red and green wavelengths, subsequently changed to light in the red and infrared wavelengths. Several years later, a US scientist, Glenn Millikan, produced a lightweight portable ear oxygen meter for use in pilots. It was Millikan who coined the term oximeter to describe his device.1 The oximeter was first commercialized in the 1960s by Hewlett Packard (Palo Alto, California) who sold a $10,000 device for use in pulmonary and sleep laboratories.
It was not until the early 1970s that 2 Japanese bioengineers, Takuo Aoyagi and Michio Kishi, serendipitously discovered that the transmission of the red and infrared frequencies of light through an earlobe showed variations corresponding with the pulsatile flow of arterial blood perfusing the tissue. They established that by measuring the pulsatile component of light transmission through living tissues they could eliminate the variable absorption of light by bone, skin, and venous circulation. They also discovered that the transmission of light in the red and infrared wavelengths could be used to calculate oxygen saturation in the arterial circulation of tissue being analyzed.2
The first pulse oximeters were manufactured in Japan in the late 1970s by Nihon Kohden and Minolta, but their clinical utility was unknown at the time. Following studies indicating that these devices could have widespread medical applications, pulse oximeters were commercialized in the United States in 1981 by Biox/Ohmeda (Ohmeda Medical; Boulder, Colorado) and by Nellcor (Covidien; Mansfield, Massachusetts) in 1983.3
The devices began to be used clinically, mostly by anesthesiologists to monitor patients undergoing sedation and anesthesia. Over the next several years, accumulated data indicated that pulse oximeters could prevent 2000 to 10,000 anesthesia deaths each year from undetected hypoxemia. In 1986, the American Society of Anesthesiologists recommended that these devices be used to monitor patients undergoing anesthesia.4 Over the next decade, pulse oximetry spread from the operating room to the emergency department, and then came to be used routinely in medical offices.
Oximetry measurements
An oximetry sensor consists of red and infrared light emitting diodes and a photodetector placed on opposite sides of a measurement site, usually the finger in adults and children but the palm or foot in neonates and toddlers. The ratio of red to infrared light that passes through the tissue depends on the percentage of oxygenated versus deoxygenated hemoglobin in the arterial circulation of the tissue. In turn, the percentage of oxygen saturation displayed by a pulse oximeter is determined by an algorithm in the microprocessor of the device based on saturation measurements obtained by sampling a large population of patients breathing mixtures of decreased oxygen concentrations.
These algorithms are unique for each manufacturer. Pulse oximeters take hundreds of readings over a 3- to 6-second time period and update their measurements every 0.5 to 1 second.2 In the best of circumstances, pulse oximeter readings come within 2% to 3% of those produced by co-oximetry, the measurement of arterial blood directly by a blood gas analyzer.
When using oxygen saturation clinically, it is important to recall the oxygen dissociation curve we learned in medical school (Figure). The upper “bend” in the oxygen dissociation curve occurs at a pO2 of 60 mm Hg of oxygen, which corresponds to an oxygen saturation of 90%. Therefore, one needs to be aware that saturation levels of 90% and below are associated with hypoxemia.
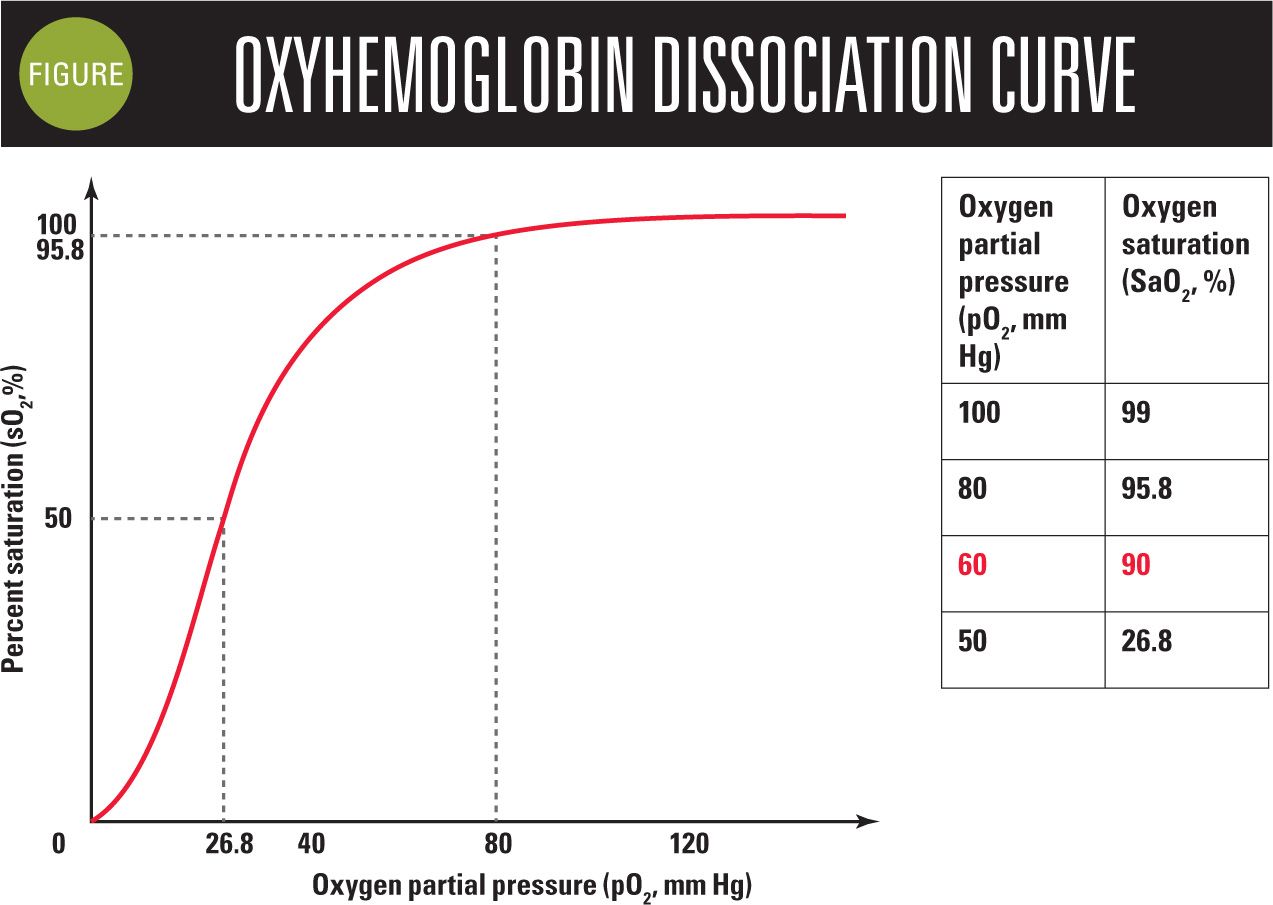
In the office, we use pulse oximetry to determine whether a patient has respiratory compromise, that is, when evaluating a child presenting with signs and symptoms of pneumonia, bronchiolitis, croup, and asthma. Pulse oximetry helps determine the severity of respiratory distress and is used to monitor how well patients respond to treatment. It should be our goal to achieve a pulse oximetry reading of 92% or higher in our patients, and to be aware of the many conditions that can interfere with oximeter readings.
Spurious oximeter measurements
There are many factors that produce spurious pulse oximetry readings. Pulse oximeters may have difficulty producing accurate measurements in cold environments, in rooms with bright fluorescent lights, and when fingernails are covered by polish (Table). Shaking from seizures or chills or just the movement from a squirming child may prevent some oximeters from detecting an adequate signal necessary to display a measurement.5
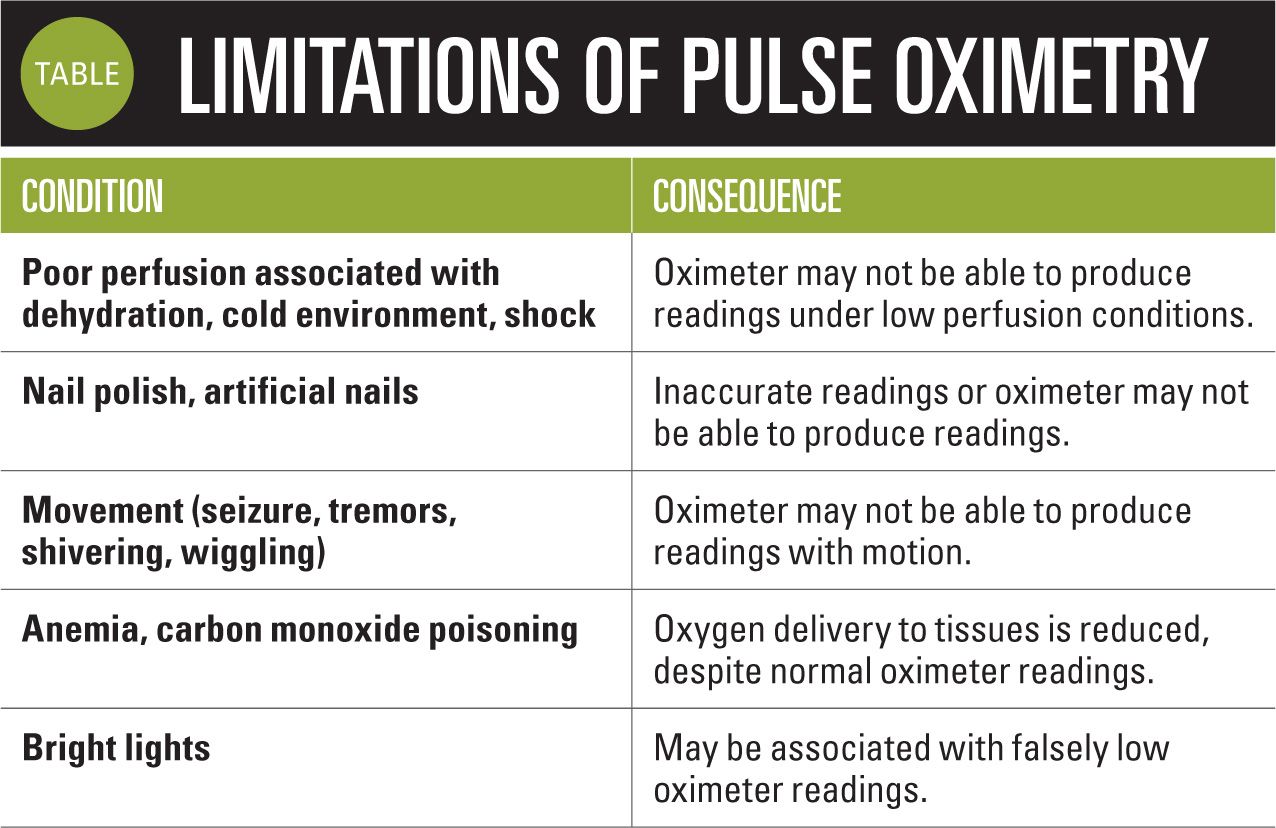
Oximeters also may have trouble acquiring a signal in patients who are significantly dehydrated or in other cases of reduced tissue perfusion, as seen in situations of anaphylactic, cardiogenic, and neurogenic shock. Keep in mind that patients with carbon monoxide poisoning will display normal saturation measurements, although the amount of oxygen being delivered to the tissues is actually reduced significantly. This is also true of patients with anemia who are likely to appear normal and display normal pulse oximeter readings, even in situations of markedly reduced hemoglobin levels, as long as perfusion remains adequate.
Advanced pulse oximetry
For many years, bioengineers realized that the movement of blood in the venous circulation in a tissue might introduce a significant degree of “noise” to the pulse oximetry readings, which may interfere with these devices displaying readings in situations of low perfusion or movement. Because of this noise, traditional pulse oximeters are subject to false alarms or inaccurate readings, often in critical situations.
In 1995, Masimo (Irvine, California) introduced an advanced method of obtaining pulse oximetry readings, using what it calls signal extraction technology (SET). I’ve described several of Masimo’s office pulse oximeters in previous Peds v2.0 articles. These devices include the traditional-appearing Pronto pulse oximeter as well as the touchscreen Pronto-7.
Many studies have demonstrated the advantages of SET in a variety of clinical situations. For example, it has been shown that Masimo’s SET pulse oximeters in the neonatal intensive care unit can reduce the incidence of retinopathy of prematurity by helping to control oxygen exposure. The technology also reduces the number of false alarms in critical care situations because SET pulse oximeters can obtain accurate readings under circumstances of low perfusion or movement.6-8 Masimo’s SET pulse oximeters and sensors were exclusively used in the 2 studies that were the basis for the Critical Congenital Heart Disease (CCHD) workgroup decision to recommend newborn screening, and the devices were the first to receive US Food and Drug Administration (FDA) 510(k) clearance with labeling for CCHD screening.9,10
Masimo pulse oximeters produce a measurement called the perfusion index (PI) that has been shown to correlate with the well-being of premature babies in the first weeks of life.11 The PI is the ratio of the pulsatile blood flow to the nonpulsatile or static blood in peripheral tissue and it represents a noninvasive measure of peripheral perfusion. Additionally, the Pronto and Pronto-7 oximeters can be used to estimate the arterial hemoglobin content (called SpHb) when used with proprietary “rainbow” sensors that use 7 separate wavelengths
of light to obtain their measurements.
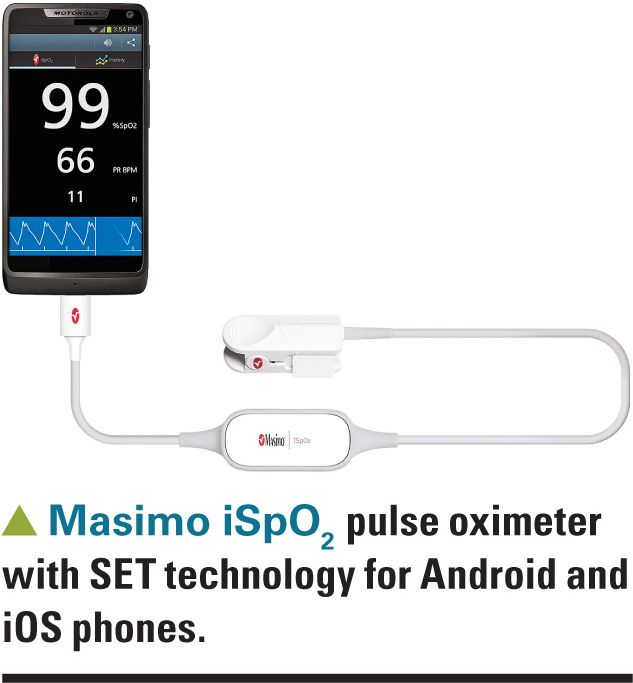
I’ve used the Pronto-7 pulse oximeter in my practice and found it useful for screening teenagers for anemia and monitoring improvement while on iron therapy. In my experience, Pronto-7 measurements are within 1 gm/dL of hemoglobin measurements obtained via traditional assays. Current SpHb sensors are too large to be used with small children, but Masimo just recently developed a sensor for use in children weighing as little as 10 kg. This new sensor is presently being evaluated by the FDA. A consumer version of Masimo’s SET pulse oximeter sensor sells for $132 (Amazon.com) and can be used with an application that runs on iOS or Android phones.
Conclusion
I hope that this article makes you appreciate the pulse oximetry technology we use every day to take the “fifth vital sign” and the efforts of the researchers responsible for its development. Pediatricians should become familiar with conditions that may interfere with pulse oximeters from providing accurate readings, and understand critical readings that correspond with hypoxemia. To obtain more dependable measurements, we should consider using devices that use SET in situations of movement and low perfusion.
REFERENCES
1. Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics. 2011;128(4);740-752.
2. Hanning CD, Alexander-Williams JM. Pulse oximetry: a practical review. BMJ. 1995;311(7001);367-370.
3. Kamat V. Pulse oximetry. Indian J Anaesth. 2002;46(4):261-268.
4. American Society of Anesthesiologists, Standards and Practice Parameters Committee. Standards for basic anesthetic monitoring. Available at: http://www.asahq.org/publicationsAndServices/~/media/For+Members/documents/Standards+Guidelines+Stmts/Basic+Anesthetic+Monitoring+2005.ashx. Approved October 21, 1986. Amended October 25, 2005. Accessed September 18, 2014.
5. Barker SJ, Shah NK. Effects of motion on the performance of pulse oximeters in volunteers. Anesthesiology. 1996;85(4):774-781.
6. Shah N, Ragaswamy HB, Govindugari K, Estanol L. Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers. J Clin Anesth. 2012;24(5):385-391.
7. Hay WW Jr, Rodden DJ, Collins SM, Melara DL, Hale KA, Fashaw LM. Reliability of conventional and new pulse oximetry in neonatal patients. J Perinatol. 2002;22(5):360-366.
8. Castillo A, Deulofeut R, Critz A, Sola A. Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 technology. Acta Paediatr. 2011;100(2):188-192.
9. Ewer AK, Middleton LJ, Furmston AT, et al; PulseOx Study Group. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378(9793):785-794.
10. de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037.
11. De Felice C, Latini G, Vacca P, Kopotic RJ. The pulse oximeter perfusion index as a predictor for high illness severity in neonates. Eur J Pediatr. 2002;161(10):561-562.
Dr Schuman, section editor for Pediatrics v2.0, is adjunct assistant professor of pediatrics, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire, and editorial advisory board member of Contemporary Pediatrics. He has nothing to disclose in regard to affiliations with or financial interests in any organizations that may have an interest in any part of this article.
Anger hurts your team’s performance and health, and yours too
October 25th 2024Anger in health care affects both patients and professionals with rising violence and negative health outcomes, but understanding its triggers and applying de-escalation techniques can help manage this pervasive issue.