Screening for hearing loss in primary care
Early intervention and ongoing surveillance are key to the management of hearing loss in childhood. Here’s why pediatricians should prioritize hearing screening at every well-child visit.
Table 1
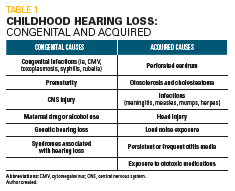
Table 2

Wispr digital otoscope
Bio-logic AuDX PRO (Natus Medical)

Biologic AuDX PRO FLEX (Natus Medical)

It is important to identify newborns with hearing loss by age 3 months so that intervention can begin by age 6 months. However, not all hearing impairment is present at birth and all infants and children should receive ongoing surveillance of hearing and communication skills at health supervision visits.1
This article will review the causes and consequences of hearing loss in childhood and describe how office-based technologies should be used to identify the hearing-impaired child. In addition, I will offer recommendations regarding the need to expedite evaluations by pediatric audiologists so that hearing-impaired children can be identified and treated in a timely fashion.
Overview of hearing loss in childhood
Childhood hearing loss is divided into 2 general categories, congenital and acquired (Table 1).
Congenital hearing loss is genetic in roughly 50% of cases. Approximately 70% to 80% of genetic hearing loss is autosomal recessive, 15% to 20% is autosomal dominant, and 2% is X-linked or mitochondrial. Fifteen percent of genetic hearing loss is part of a syndrome, such as Usher syndrome and Waardenburg syndrome, and more than 400 syndromes are associated with hearing loss.1 Nongenetic causes include prematurity, central nervous system injury, drug/alcohol use by mothers during pregnancy, and those caused by congenital infections.2
Acquired hearing loss is a consequence of various circumstances that can affect children, including perforated eardrums, protracted otitis media, otosclerosis, and cholesteatomas. Additional causes include prolonged and repetitive exposure to loud noises, exposure to ototoxic medications, infections, and head injury.1
Another way to view childhood hearing loss is to consider the anatomy involved. Conductive hearing loss involves problems between the external auditory canal and the cochlea, including vernix in newborns, cerumen in children, as well as aural atresia and otitis media. Sensory hearing loss is a consequence of cochlear dysfunction that may result from genetic causes, or exposure to ototoxic medications or infections. Neural causes of hearing loss include failure of function of the auditory nerve or disruption of auditory processing in the brain. Causes may include tumors, bleeding (intrapartum, postpartum or central nervous system [CNS] injury in childhood), infections, and auditory neuropathy.2
Incidence and consequences of hearing loss
As with any childhood-related medical issue, it is important for providers to be aware of the incidence of hearing impairment in childhood so the problem can be put into perspective and given an appropriate priority for screening during well visits.
According to data from the Centers for Disease Control and Prevention (CDC) from 2017, approximately 98% of newborns in the United States are screened for congenital hearing loss.3 The data indicate that 1.7 of every 1000 babies in the United States is born with hearing loss, and 25% of babies do not undergo follow-up testing (ie, are lost to follow-up). Neonatal intensive care unit (NICU) patients are at higher risk for neurosensory hearing loss with an incidence of 16.7/1000 infants and an auditory neuropathy incidence of 5.6/1000 infants.4,5
According to some estimates, the incidence of childhood hearing loss increases to 6 of 1000 children in the United States by age 6 years.2 The National Health and Nutrition Examination Surveys indicate that hearing loss greater than 25 dB affects 3% to 5% of American adolescents!6
The sooner a hearing impairment is identified in an infant or child, the sooner the child can receive hearing aids or, when appropriate, a cochlear implant as well as educational support should the family decide to adopt these options. Without detection of hearing impairment, even a moderate impairment, the child may perform poorly in school, develop a poor self-image, and consequently fail to achieve their potential. Numerous studies have shown that the sooner a hearing-impaired child receives hearing augmentation, the sooner they develop vocal communicative and linguistic skills.7,8
When to be concerned
Primary care providers should be suspicious that a child may have a significant hearing loss if a child has a speech or developmental delay or if parents are concerned about a possible hearing loss. Other aspects of the history that should prompt consideration of an audiology referral include a family history of childhood hearing loss, neonatal intensive care of 5 days or longer, in utero infections, craniofacial anomalies, and other risk factors listed in Table 2.
Technologies for screening in primary care
The Joint Committee on Infant Hearing (JCIH) updated its screening guidelines last year. The JCIH recommends 2 separate hearing screening protocols: 1) for those admitted to a NICU for more than 5 days; and 2) for babies born in well nurseries.1
All NICU babies (representing 10% of the entire newborn population) may be at risk for neurosensory hearing loss and auditory neuropathy and should undergo hearing screening using an automated auditory brainstem response (AABR) test prior to discharge. Infants cared for in the NICU who do not pass the AABR should be referred directly to an audiologist for rescreening and, when indicated, given a comprehensive audiologic evaluation including diagnostic ABR.
In the well nursery, babies can undergo a screening using either AABR testing or otoacoustic emissions (OAE) testing with a rescreening by the hospital as outpatient with either technology. Alternatively, newborns in the well nursery can undergo an initial screen with either technology with a rescreen before discharge with either technology should the baby not pass the initial screen. All babies who refer from the well nursery should be rescreened by the hospital before aged 1 month or rescreened in the pediatrician’s office (using OAE devices, see below). If this is not possible, they should be referred to a pediatric audiologist for follow-up. It is the goal of Universal Newborn Hearing Screening (UNHS) that screening is completed by age 1 month, audiologic diagnosis is completed by age 3 months, and that the hearing-impaired child is enrolled in early intervention by age 6 months.1
Primary care providers have several tools at their disposal for screening young children for hearing loss. According the American Academy of Pediatrics (AAP) Bright Futures Guidelines, children should be screened for hearing loss at ages 4 ,5, 6, 8, and 10 years; once between the ages of 11 and 14 years; once between ages 15 and 17 years; and once between the ages of 18 and 21 years.9 Screening performed at age 10 years or younger usually is done at 2000 to 5000 Hz. Older children also should be screened at 6000 to 8000 Hz to detect high-frequency hearing loss. Screening also should be performed any time a provider is concerned about hearing loss, as would be the case with children presenting with delayed speech or those with prolonged or recurrent otitis media. In an office environment, OAE screeners are affordable and screening can be completed in minutes.
In the office setting, those children who refer on OAE screening should have an in-office tympanometry test (or screening with acoustic otoscope, see below) to exclude middle-ear effusion as a cause of the hearing loss. As OAE screeners generate a pass or refer result, pediatricians should consider performing a pure tone audiogram to determine the hearing threshold for the child. Be aware that 20% of 3- to 5-year-old children will not be able to be screened by pure tone audiometry and those unable to be screened should be evaluated by an audiologist.
Hearing screeners such as the Bio-logic AuDX PRO and Bio-logic AuDX PRO FLEX from Natus Medical (Pleasanton, California) are modular devices that facilitate screening in the primary care office. The Bio-logic AuDX PRO can be configured to display a cartoon to distract a child during the brief OAE screen. Additionally, it can be used to perform pure tone audiometry on children who refer on OAE screen or for those suspected as having auditory neuropathy. The Biologic AuDX PRO FLEX provides all the capabilities of the AuDX PRO as well as providing tympanometry capability. In the latter situation, one device provides all the hearing screening capability a practice needs.
Every child with hearing loss should have a visual inspection of the tympanic membrane and ear canal. Cerumen impaction, serous otitis, acute otitis, tympanic membrane perforation, otosclerosis, and cholesteatoma can contribute to conductive hearing loss. The inspection can be done with a standard otoscope using pneumatic otoscopy to check the mobility of the ear drum. In the real world, pneumatic otoscopy is rarely employed by pediatricians. Otoscopy also can be difficult in an uncooperative child or when cerumen obscures the view of canal and tympanic membrane.
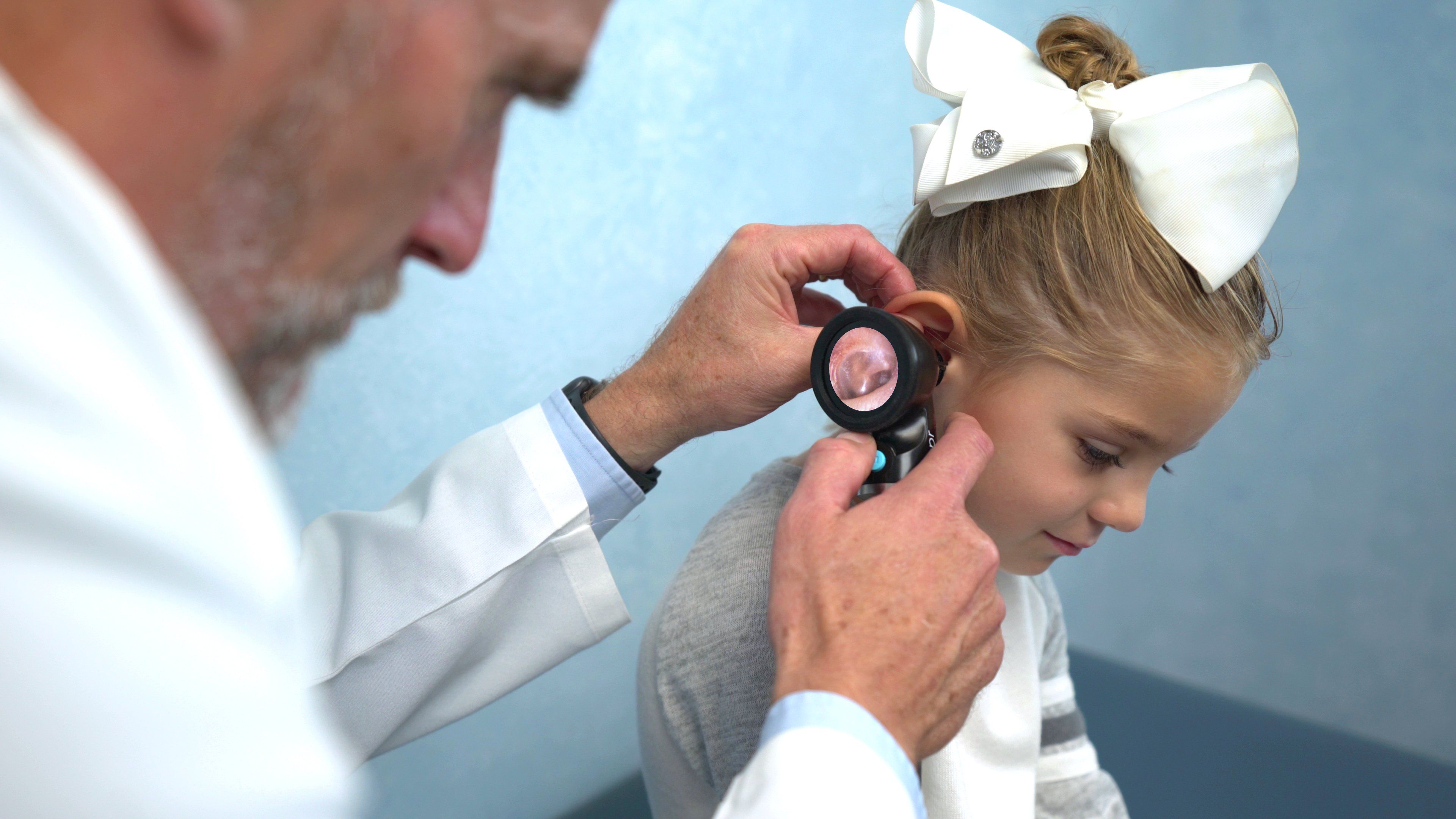
The Wispr Digital Otoscope from WiscMed (Madison, Wisconsin) allows one to insert a small speculum into the ear canal while guiding insertion by looking at the viewing screen. One can then capture images or select images from a video and share with parents. These videos can be imported into the medical record and shared with audiologists and otolaryngologists (ENTs), and used for comparison purposes when one decides to monitor the resolution of a perforation or otitis.
Per the guidelines issued by the AAP in 2013, one needs to visualize a bulging tympanic membrane or intense erythema of the tympanic membrane in addition to documenting the presence of fluid in the middle ear space to diagnose otitis media.10 Assessment of fluid in the middle-ear space can be done with pneumatic otoscopy or with tympanometry. Both require a cooperative child in order to obtain and maintain a seal between the speculum or probe and the wall of the ear canal.
The Acoustic Otoscope from Check My EAR LLC (Lincoln, Nebraska) is expected to become available by mid-2020. It enables providers to determine if there is fluid in the middle-ear space in a matter of seconds. No seal is required and cerumen usually does not interfere with measurements (see “Must have gadgets for today’s pediatrician,” Contemporary Pediatrics, December 2019). Additionally, the Acoustic Otoscope provides a spectral gradient angle measurement that can be followed at subsequent visits to monitor the resolution of ear effusions.
There are 2 new devices that will be released in the near future that will facilitate diagnosis and monitoring of otitis media. I plan on reporting on these in my December 2020 “best tech” article for Contemporary Pediatrics.
Audiologist and ENT referrals
Pediatricians should not delay referral of children with suspected hearing loss to audiologists. Audiologists with expertise in treating children have a range of methods to determine hearing thresholds in young children. These include Behavioral Observation Audiometry, Play Audiometry, and Visual Reinforcement Audiometry.11
Part of the evaluation includes tympanometry and a determination whether hearing loss is conductive or central. When appropriate, an audiologist will refer to an ENT physician for further evaluation and generate a referral to a medical facility that can perform an ABR diagnostic study. The earlier a child is identified with hearing loss, the sooner the affected child can receive educational services and hearing augmentation when indicated. It is therefore important to establish a relationship with audiologists in your community and make an appointment for the patient before the patient leaves the pediatrician’s office.
In conclusion
It is imperative that pediatricians ensure that all patients in our care undergo appropriate screening for hearing loss and that we remain diligent in identifying patients who may be at risk for developing permanent hearing loss in childhood. As discussed, we should utilize appropriate equipment to screen children for hearing impairment and determine if there is fluid in the middle ear. Lastly, we should err on the side of caution and refer to an audiologist promptly when hearing loss is suspected.
References:
1. Joint Committee on Infant Hearing. Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. JEHDI. 2019;4(2):1-44.
2. Todd NW. The etiologies of childhood hearing impairment. In: NCHAM E-Book: A Resource Guide for Early Hearing Detection and Intervention. National Center for Hearing Assessment and Management; 2015; ch 6. Accessed April 8, 2020. http://www.infanthearing.org/ehdi-ebook/2015_ebook/6-Chapter6Etiologies2015.pdf
3. Centers for Disease Control and Prevention. 2017 Annual Data Early Hearing Detection and Intervention (EHDI) Program. Last reviewed December 5, 2019. Accessed April 8, 2020. https://www.cdc.gov/ncbddd/hearingloss/ehdi-data2017.html
4. Xoinis K, Weirather Y, Mavoori H, Shaha SH, Iwamoto LM. Extremely low birth weight infants are at high risk for auditory neuropathy. J Perinatol. 2007;27(11):718-723.
5. Korver AM, van Zanten GA, Meuwese-Jongejeugd A, van Straaten HL, Oudesluys-Murphy AM. Auditory neuropathy in a low-risk population: a review of the literature. Int J Pediatr Otorhinolaryngol. 2012;76(12):1708-1711.
6. Barret TS, White KR. Trends in hearing loss among adolescents. Pediatrics. 2017;140(6):e20170619.
7. Robinshaw HM. Early intervention for hearing impairment: differences in the timing of communicative and linguistic development. Br J Audiol. 1995;29(6):315-334.
8. Apuzzo ML, Yoshinaga-Itano C. Early identification of infants with significant hearing loss and the Minnesota Child Development Inventory. Semin Hear. 1995;16:124-139.
9. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
10. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
11. Sabo DL. Assessment of the young pediatric patient. In: The NCHAM E-Book:A Resource Guide for Early Hearing Detection and Intervention. National Center for Hearing Assessment and Management; 2015:ch 5. Accessed April 8, 2020. https://www.infanthearing.org/ehdi-ebook/2015_ebook/5-Chapter5Assessment2015.pdf