Smallpox: Preparing for an old (but no longer familiar) threat
Smallpox has re-emerged--not yet as a disease but as a subject of apprehension, debate, and misunderstanding. Here, an expert reviews the disease, vaccine, and proposed immunization strategies.
Cover article
SMALLPOX:
Preparing for an old (but no longer familiar) threat
By Robert S. Baltimore, MD
Once thought to be gone forever, smallpox has re-emerged not yet as a disease but as a subject of apprehension, debate, and misunderstanding. Here, an expert reviews the disease, vaccine, and proposed immunization strategies.
Fear about terrorism swept the United States after the intentional plane crashes into the World Trade Center and the Pentagon on September 11, 2001, and the mailing of anthrax-laced letters the following month. One specific concern that arose was that the smallpox (variola) virus could be used as another agent of bioterrorism.
Misinformation about smallpox is common, according to the findings of a recent study.1 People are misinformed about whether the disease still exists, about how protective the vaccine is, and about whether smallpox can be treated effectively.1 Answers to questionnaires given to physicians by the Centers for Disease Control and Prevention (CDC) demonstrate that some physicians, too, are misinformed about smallpox.
This review examines smallpox disease, the threat of smallpox virus as an intended agent of disease, and the controversial issue of what place smallpox vaccine should have in the nation's response to this threat.
Ancient times to present day
Smallpox is a communicable disease caused by variola virus that has been known since antiquity. It is estimated to have caused 500 million deaths worldwide in the 20th century. In 1967, the year efforts were first made to eradicate smallpox, the disease killed 2 million people around the globe.
Variola virus is a member of the Orthopoxviridae family and is found in two varieties: one that causes variola major (severe smallpox) and the other, variola minor (a less severe variant). Other orthopoxviruses include vaccinia, the virus with which the present-day smallpox vaccine is formulated; cowpox, the virus that Edward Jenner first used to inoculate people against smallpox in 1796; and monkeypox, a rare but serious disease of monkeys in Africa that occasionally spreads to humans.
Efforts of the World Health Organization led to eradication of the natural reservoir of smallpox virus and the disease in 1978. Until then, mass vaccination programs had been effective at reducing the rate of smallpox but did not work in very isolated rural populations in the developing world. "Ring vaccination" ("search and containment"), in which cases are identified by health-care workers, and then all direct contacts, and contacts of these contacts, are immediately vaccinated, eventually resulted in eradication (Figure 1).

The last case of smallpox in the US was in 1949; the last naturally occurring case in the world was in Somalia in 1977; and the last known caseassociated with a laboratorywas in Birmingham, England, in 1978. After two years, during which no new cases were found, smallpox was officially declared eradicated in 1980, and laboratories were told to destroy their supply of variola. The only official stocks of virus are kept at the CDC in Atlanta, Georgia, and at the Research Institute for Viral Preparations in Moscow, Russia.
Routine use of smallpox vaccine in the US ended in 1972 because the risk of serious side effects was considered greater than the risk of exposure to variola, which had almost disappeared. In 1983, the vaccine was removed from the commercial market and production ceased. Vaccination of military personnel was discontinued in 1990. Until recently, vaccination was recommended only for laboratory personnel who could be exposed to orthopoxviruses and for researchers working with vaccinia virus.2,3
Today, teams of physicians from the CDC with expertise in the management of smallpox and teams of health-care workers who would care for a patient with smallpox in a hospital are being vaccinated. As of late March 2003, approximately 26,000 civilians had been vaccinated, as well as an estimated 500,000 military personnel.
The often fatal course of smallpox
Variola is usually spread in droplets or an aerosol from the oropharynx of an infected person. Direct contact with infected skin lesions or with clothing or bed linens contaminated with the virus can also transmit the disease. The incubation period is seven to 17 days (mean, 12 days), after which infectivity begins with an enanthema and a rash on the face and extremities. Most patients feel ill enough during the prodromal phase to require complete bed rest by the time the rash appears; fever develops two to four days before the rash does.
The classic rash develops in stages, and all lesions are at the same stage of development at once. The rash begins as maculae, then progresses to papules. By about days 2 to 4 of the rash, there are deep, tense vesicles; by approximately days 4 to 5, the vesicles turn to round, tense, deep pustules (Figure 2). The pustules dry to scabs by about days 9 to 10, after which the scabs separate. The period of infectivity continues until the scabs have been shed.
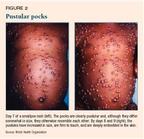
Patients who do not improve usually become toxic during the second week of illness, developing an overwhelming generalized illness. Older epidemiologic data show a mortality rate of approximately 30% for patients infected with smallpox. Infants, the elderly, pregnant women, and immunodeficient persons (including those on immunosuppressive therapy and those who are infected with human immunodeficiency virus) have a less favorable prognosis.
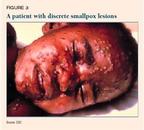
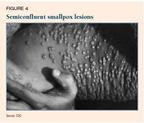
The number and density of lesions is an important prognostic finding. Mortality among patients who have discrete lesions (Figure 3) is less than 10%; among patients who have semiconfluent lesions (Figure 4), 25% to 50%; and among those who have confluent lesions (Figure 5), 50% to 75%.

Approximately 90% of patients have the classic rash; the rest develop a variant (Table 1). The flat, or malignant, variant is uncommon (7% of patients); these patients have more sudden and severe onset of disease, often with severe abdominal pain, and their rash never forms pustules or scabs. More than 90% of people who have this variant die. In the hemorrhagic variant, which is rare (2% of patients), the prodrome is also more acute and severe, and is followed by a bleeding diathesis before the onset of rash; mortality is 100%.4,5
TABLE 1
Variants of the smallpox rash
Discrete
Semiconfluent
Confluent

Patients with variola minor have fewer constitutional symptoms than those with variola major, and a rash with relatively sparse lesions.
The cardinal features of smallpox that differentiate it from varicella (chickenpox) are that, in smallpox, the lesions are all in one stage of development (Figure 2); in chickenpox, lesions in all stages of development are seen at once. In smallpox, the early rash is most intense on the face and extremities; in varicella, the early rash is most intense on the trunk.6 Table 2 lists distinguishing features.
TABLE 2
Comparing the rashes of smallpox and varicella
Complications of smallpox include secondary bacterial infections of the skin, eyes, and respiratory tract, which can result in septicemia and disseminated bacterial disease; laryngeal lesions, which can cause edema and airway obstruction; and encephalitis.
Renewed cause for concern?
As mentioned, supplies of smallpox virus remain in government laboratories in the US and Russia. Reportedly, the former Soviet Union was developing smallpox as a biological weapon. Although natural smallpox is transmitted person to person, fears exist that the Soviets may have developed the means to spread the virus in man-made aerosols. With Russian government funding for its laboratories declining in recent years, concern has arisen that the virus, along with the expertise to propagate a large amount of it, has fallen into unauthorized hands outside the Russian government.2,7,8
Several characteristics of smallpox make it a possible agent of bioterrorism:
- It can be produced in large quantities.
- It can be made stable for storage and transportation, and could possibly be made stable for use as an aerosol.
- It is likely to disrupt society because it is associated with high mortality, the rate of person-to-person spread is relatively high, and, at this time, most of the world has little or no immunity.
How might smallpox be introduced by an act of bioterrorism? There are a number of possible scenarios, and experts disagree on which one is most likely. It is possible that one or more people would be deliberately infected and sent to the US or other Western country during their period of contagion. They might thus infect people in an airplane, airport, or other congregate setting. The argument against this scenario is that, during the period of contagion, a person is very ill and might be detected because of their rash or obvious malaise and fever. In the past, such outbreaks were prolonged because of spread in hospitals from patients to hospital personnel or to other patients. Today, appropriate airborne isolation technique should limit such transmission.
Introduction of smallpox virus by an artificial aerosol is another possibility and is the method of delivery that could cause the most harm. Although it is suspected that scientists in the former Soviet Union once worked on this method of bioweapons technology, evidence that any suspected bioterrorist actually has the capability to carry out such an attack is lacking.
There has been no official estimate of how likely it is that smallpox will be introduced into the US population. Many bioterrorism experts believe that it is quite unlikely.
Immunization and treatment
In the past, all children were immunized with vaccinia virus starting at 1 year of age. Immunization stimulates an immune response that cross-reacts with variola and protects the vaccine recipient. A person's concentration of neutralizing antibodies decreases significantly over the next five to 10 years, so anyone vaccinated before 1972 is unlikely to be fully protected against smallpox today. Antibodies and cell-mediated immunity may, however, protect against death from smallpox for more than 30 years.
No known antiviral agent is effective against smallpox, although some experts speculate, based on in vitro data, that cidofovir may offer benefit. However, the drug is not FDAapproved for this use. Patients should receive supportive care, including, when appropriate, hydration and treatment of secondary bacterial infection. Primary and secondary contacts of persons infected with smallpox should be vaccinated as soon as possible. Postexposure immunization affords considerable protection against smallpox, particularly against death from the disease. Vaccination within three to four days of exposure will prevent or largely modify the severity of smallpox. Vaccination up to seven days after exposure will probably modify the severity of disease.
Smallpox vaccine: Available? Safe?
The CDC stores smallpox vaccine in a frozen lyophilized state. The approximately 15 million doses of Dryvax (Wyeth) that exist are enough to vaccinate the military and control a possible outbreak, but not enough to immunize the whole US population. The vaccine was made by first inoculating the virus (vaccinia) onto calf hide, then using the "lymph" produced from the resulting lesion. Data suggest that the vaccine may be diluted at least 1:5 to 1:10 and still be effective.3,9,10
A previously undisclosed supply of about 85 million doses of concentrated smallpox vaccine that had been put aside by Aventis Pasteur remains biologically active and has been transferred to the CDC. It is similar to Dryvax but is in liquid form. After dilution of this vaccine, the supply of vaccine would be sufficient to immunize the entire US population.11,12
A new vaccine being developed by Acambis Baxter is made from the same vaccinia strain but is produced in tissue culture. Unlike calf lymph vaccines, it is expected to be free of microbial contaminants and a purer product. It is unknown whether it will be safer than the existing vaccine.
The current vaccinia virus vaccine is inoculated into the deltoid area of the upper arm or the lateral area of the lower leg with a bifurcated needle; a series of jabs forces a drop of the vaccine beneath the epidermis. When immunization is successful ("take"), a papule develops at the vaccination site. This lesion matures into a pustule and then a scab that separates by approximately the third week after vaccination (Figure 6).13 Vaccinia virus can be recovered at each stage, including from the scab that separates.9 Reimmunization usually causes a milder lesion that evolves more quickly.
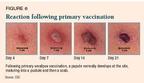
Immunization can also cause a local infection that is pruritic and uncomfortable, and is often accompanied by fever, malaise, and regional lymphadenitis about a week after immunization. A recent report found that 36% of vaccinees were sufficiently ill to miss school, work, or recreational activities or to have trouble sleeping.10
Complications of immunization can be serious and include death, postvaccinal encephalitis, progressive vaccinia, eczema vaccinatum, generalized rash, and autoinoculation of another part of the body after scratching the immunization lesion (Table 3). The risk of a severe complication is the main reason not to resume universal immunization at a time when not a single case of smallpox has actually occurred. The risk is particularly high for the immunocompromised.
TABLE 3
Smallpox vaccine adverse event rates in the United States, 1968
A person with a chronic skin condition who receives the vaccine is at risk of eczema vaccinatum, a severe dermatologic involvement that is sometimes fatal. Atopic dermatitisa genetically based immune disorder that occurs within the first 5 years of life and affects 15% of the population14is the most common condition associated with severe eczema vaccinatum; these patients may be at risk even if their dermatitis is in remission. Moreover, patients with a chronic skin condition who do not get immunized can still develop eczema vaccinatum if they are exposed to vaccinia virus from someone who has been vaccinated. Smallpox vaccine is contraindicated, therefore, in people with eczema or other exfoliative skin disorders. It is also contraindicated in women who are pregnant or breastfeeding and in people with a primary or secondary immunodeficiency. Table 4 lists these and other specific contraindications for both potential vaccinees and close contacts.
TABLE 4
Contraindications to smallpox vaccination in the absence of an exposure to smallpox
Shortly before this article went to press, the CDC announced that it had received reports of more than a dozen adult vaccinia vaccinees who had developed a serious cardiac adverse event after vaccination. The possible link between smallpox vaccination and these adverse events is being investigated. In the meantime, people with a history of heart disease will not be vaccinated against smallpox.
Studies from the 1960s indicate an overall rate of complications among primary (first-time) vaccinees of 1,254 for every 1 million.1518 Vaccinia immune globulin (VIG) was used to treat many of these complications. Based on 1968 US data, estimates are that at least 40 people for every 1 million immunized will develop a significant and potentially life-threatening complication for which VIG will be needed. Given that only about 600 doses of VIG are available, and that more people today are immunosuppressed than were in the 1960s, our supply of VIG is inadequate. More VIG is being produced under government contract.
Vaccine policies: Weighing the options
Following the terrorist events of September and October 2001 and the recognition that smallpox virus might be used as an agent of bioterrorism, many experts and other commentators publicly voiced their opinion about smallpox vaccination. Major opinions varied: Some recommended mass vaccination; some, voluntary vaccination; others, vaccination of "first responders" only; and still others, vaccination only after smallpox had actually been introduced.12 Arguments for each recommendation can be summed up succinctly.
Proponents of mass vaccination cite this as the most effective way to prevent transmission of disease and thereby protect the population if the introduction of smallpox overwhelmed the health-care community.19,20 They also postulate that it is unlikely a bioterrorist would introduce vaccinia into a population that is largely vaccinated. Although this rationale might be valid, such a policy would also expose the largest number of people to the risks of vaccination even though the likelihood of exposure to smallpox and the subsequent risk of disease might in fact be nil. In addition, with so many people having a contraindication to vaccination, there might be so many unvaccinated people as to not pose a deterrent to the spread of the virus.
Those who support voluntary vaccination believe that people should be allowed to individually consider the advantages and disadvantages of vaccination and act on the basis of their own analysis of risk and benefit.12,21 Proponents of this policy assume that people will be sufficiently familiar with that risk and benefit to make a reasoned decision. Regrettably, a large part of the population is unfamiliar with the issues surrounding vaccinia immunization; public information polls have demonstrated a significant number of false beliefs about the disease and the vaccine among the general population and health-care workers.1 When people are given correct information, resistance to receiving the vaccine increases.
A policy in which vaccination is carried out only when smallpox is introduced is favored by those who believe that ring vaccination would be the safest and most effective strategy.22 The American Academy of Pediatrics (AAP) favors this strategy.23 The major problem with such a policy is whether the public would accept a public health response in which only a limited population would initially be vaccinated after smallpox was introduced. Those who argue against this policy say the public health infrastructure could not, in a timely fashion, meet the immediate nationwide demand for vaccine that would ensue. The AAP recognizes the possibility that expansion of the ring of vaccine recipients may be required if there is more substantial evidence of a bioterrorism threat.
A strategy that emphasizes vaccination of first responders is a component of current US policy.24 First responders are not rigorously defined, but generally include hospital-based personnel who would be required to assess and care for people with smallpox. This includes emergency department personnel, diagnostic imaging technicians, nurses, critical care specialists, and certain other specialists such as pediatricians, infectious diseases specialists, dermatologists, and selected support personnelhousekeeping and laboratory technicians, for example. The concept could also be applied to emergency medical technicians, police, and many others.
Initially, the Advisory Committee on Immunization Practices of the CDC defined this group rather narrowly and estimated that 15,000 to 20,000 health-care personnel would need to be vaccinated.25 Subsequently, the federal government expanded that number to as many as 500,000 in the initial stage.24,26
Federal policy recommends a three-stage vaccination strategy:
- Phase 1. A limited number of first responders and public health investigation teams are vaccinated. (Only about 26,000 volunteers have been vaccinated in this phase so far.)
- Phase 2. Widespread immunization of health-care workers (perhaps as many as 10 million vaccinees) is undertaken.
- Phase 3. All citizens who do not have a contraindication can submit to voluntary vaccination.
Phase 3 would not commence until 2004, at the earliest. In addition, the CDC has developed a plan for immediate mass vaccination that would occur only in the event of a verified smallpox threat.27
REFERENCES
1. Blendon RJ, Desroches CM, Benson JM, et al: The public and the smallpox threat. N Engl J Med 2003; 348:426
2. Henderson DA, Inglesby TV, Bartlett JG, et al: Smallpox as a biological weapon: Medical and public health management. JAMA 1999;281:2127
3. Breman JG, Henderson DA: Diagnosis and management of smallpox. N Engl J Med. 2002;346;1300
4. Center for the Study of Bioterrorism. Saint Louis University School of Public Health, accessed at http://www.slu.edu/colleges/sph/csbei/bioterrorism/smallpox.htm
5. Koplan JP, Foster SO: Smallpox: Clinical types, causes of death, and treatment. J Infect Dis 1979;140:440
6. World Health Organization Slideset on Smallpox. Accessed at http://www.who.int/emc/diseases/smallpox/slideset/pages/spox_031.htm
7. Alibek K: Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World, Told From the Inside by the Man Who Ran It. New York, Random House, 1999
8. O'Toole T: Smallpox: An attack scenario. Emerg Infect Dis 1999;5:540
9. Frey SE, Newman FK, Cruz J, et al: Dose-related effects of smallpox vaccine. N Engl J Med 2002:346:1275
10. Frey SE, Couch RB, Tacket CO, et al: Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med 2002;346:1265
11. Pear R: The bioterrorism threat. Frozen smallpox vaccine is still potent, officials say. The New York Times. March 30, 2002, page A8
12. Fauci AS: Smallpox vaccination policyThe need for dialogue. N Engl J Med 2002;346:1319
13. Rubins K, Relman DA: Progression of the lesion at the site of inoculation after smallpox vaccination. N Engl J Med 2003:348:414
14. Rudikoff D, Lebwohl M: Atopic dermatitis. Lancet 1998;351:1715
15. Centers for Disease Control and Prevention: Smallpox vaccination and adverse reactions. MMWR Dispatch January 24, 2003;52:1
16. Lane JM, Ruben FL, Neff JM, et al: Complications of smallpox vaccination, 1968. N Engl J Med 1969; 281:1201
17. Lane JM, Ruben FL, Neff JM, et al: Complications of smallpox vaccination, 1968: Results of ten statewide surveys. J Infect Dis 1970;122:303
18. Lane JM, Millar JD: Risks of smallpox vaccination complications in the United States. Am J Epidemiol 1971;93:238
19. Kaplan EH, Craft DL, Wein LM: Emergency response to a smallpox attack: The case for mass vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2002; 99:10935
20. Bozzette SA, Boer R, Bhatnagar V, et al: A model for a smallpox-vaccination policy. N Engl J Med 2003; 348:416
21. Bicknell WJ: The case for voluntary smallpox vaccination. N Engl J Med 2002;346:1323
22. Mack T: Sounding board. A different view of smallpox and vaccination. N Engl J Med 2003;348; 460
23. Abramson JS, Baker CJ, Baltimore RS, et al: Smallpox vaccine. Pediatrics 2002;110:841
24. Centers for Disease Control and Prevention: Protecting Americans: Smallpox Vaccination Program. Accessed at http://www.bt.cdc.gov/agent/smallpox/vaccination/vaccination-program-statement.asp
25. Centers for Disease Control and prevention: Draft Supplemental Recommendations of the ACIP: Use of Smallpox (Vaccinia) Vaccine, June 2002. Accessed at http://www.bt.cdc.gov/agent/smallpox/vaccination/acip-guidelines.asp
26. The White House: Frequently Asked Questions. Smallpox Response Teams. Accessed at http://www.whitehouse.gov/news/releases/2002/12/0021213-3.html
27. Centers for Disease Control and Prevention: Smallpox vaccination clinic guide. September 16, 2002. Accessed at http://www.bt.cdc.gov/agent/smallpox/response-plan/files/annex-3.pdf
DR. BALTIMORE is professor of pediatrics and epidemiology, Yale University School of Medicine, New Haven, Conn., and attending physician and associate hospital epidemiologist, Yale-New Haven Hospital and Yale-New Haven Children's Hospital. Dr. Baltimore is a lead author of the American Academy of Pediatrics policy statement on smallpox vaccine.
Precautions to take if you are vaccinated against smallpox
Many physicians want to know whether they should cease contact with patients if they themselves receive the smallpox vaccine. According to the Centers for Disease Control and Prevention, taking a leave from one's practice is not usually required for newly vaccinated health-care workers unless they meet any of the following conditions:
- They are physically unable to work because of systemic signs and symptoms of vaccine-related illness.
- They have extensive skin lesions that cannot be adequately covered.
- They do not adhere to the recommended infection control precautions.
It is important to realize that the very close contact required for transmission of vaccinia to household contacts is unlikely to occur in the health-care setting.
The CDC offers this advice to physicians and other health-care workers who have been vaccinated against smallpox:
When at work, cover the vaccination site loosely with gauze, using first-aid adhesive tape to keep it in place. Then cover the gauze with a semipermeable (or semiocclusive) dressing such as Opsite or Tegaderm. (A semipermeable dressing is one that does not allow passage of fluids but does allow passage of air.) Change the gauze and dressing every one to three days, ideally, and every three to five days at a minimum, to prevent build-up of fluids and irritation of the vaccination site until the dried scab finally separates from the skin. (After I was vaccinated, I found that I needed to change the bandage more frequently in the pustular phase, when there was more exudate, and less often when the scab had dried.) As an added precaution, wear a shirt that covers the vaccination site well. It is a good idea to have a colleague inspect the site at the beginning of every day that you will be seeing patients to make sure that no uncovered lesions, severe dermatologic reactions, or satellite or autoinoculation lesions are present.
Wash your hands with soap and warm water or with alcohol-based hand rubs, such as a gel or foam, after direct contact with the vaccine, the vaccination site, the bandage, or anything that might be contaminated with live virus, including clothing, towels, or sheets that came in contact with the vaccination site. This is vital to remove any virus from your hands and prevent spread by contact. Keep the vaccination site dry. Cover the vaccination site with a waterproof bandage when you bathe.
Robert S. Baltimore, MD
For more information
Centers for Disease Control and Preventionwww.bt.cdc.gov/agent/smallpox
World Health Organization www.who.int/csr/disease/smallpox/en
American Academy of Pediatricswww.aap.org/terrorism/index.html
Infectious Diseases Society of America
www.idsociety.org/bt/toc.htm
Saint Louis University School of Public Health, Center for the Study of Bioterrorism
www.slu.edu/colleges/sph/csbei/bioterrorism/smallpox.htm
Robert Baltimore. Smallpox: Preparing for an old (but no longer familiar) threat. Contemporary Pediatrics May 2003;20:42.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.