Tapinarof cream 1% demonstrates positive long-term results for atopic dermatitis in patients 2 years and up
The newest tapinarof results were presented at the 2025 American Academy of Dermatology (AAD) Annual Meeting.
Image Credit: © Elroi - stock.adobe.com.
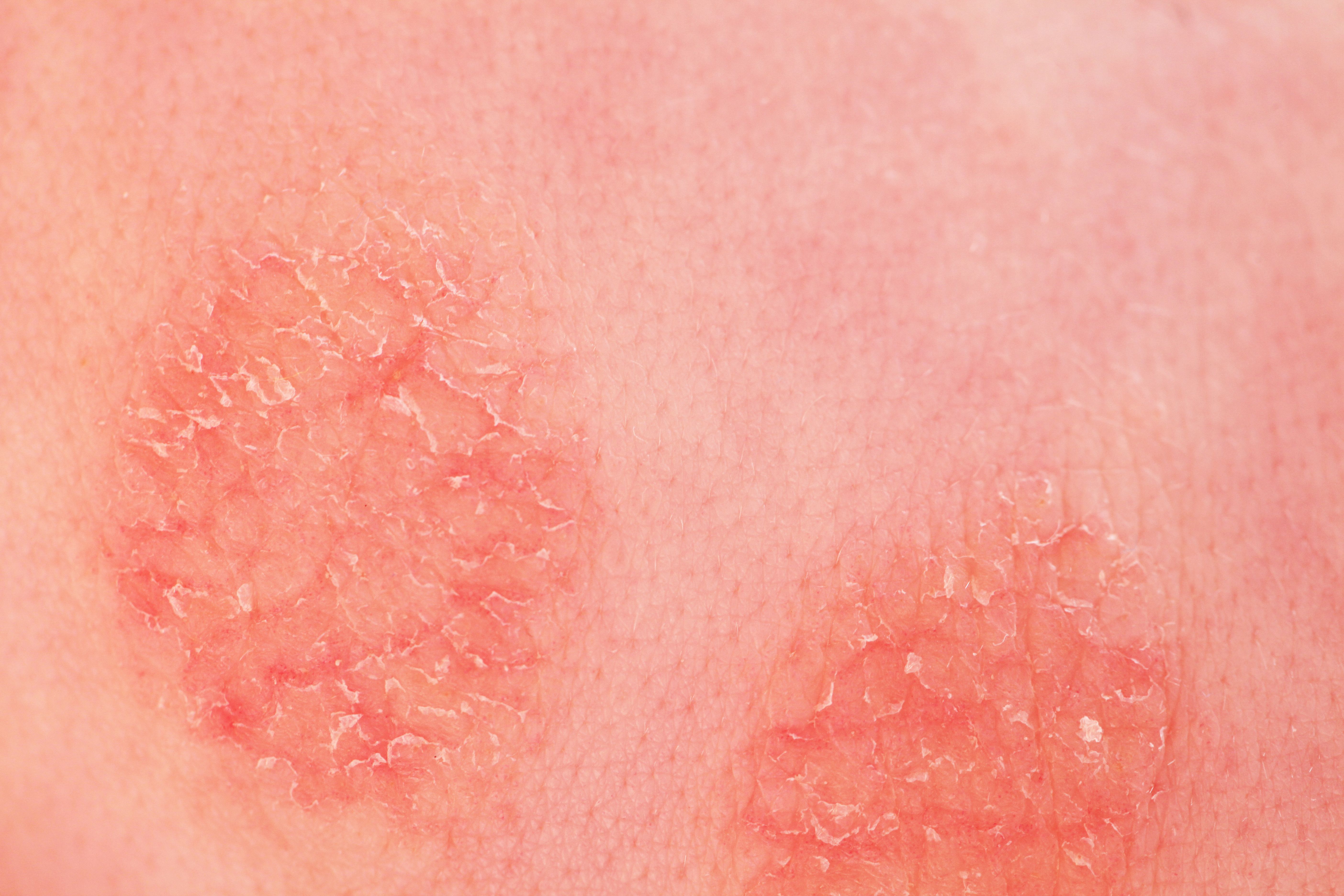
Organon has recently announced new results from the phase 3 ADORING 3 open-label, long-term extension study evaluating tapinarof cream, 1% (VTAMA), for the treatment of atopic dermatitis (AD). The findings were presented during a late-breaking research session at the 2025 American Academy of Dermatology (AAD) Annual Meeting.1
The ADORING 3 study demonstrated that AD disease activity remained mild in patients who had achieved treatment success and then entered a treatment-free interval, which lasted an average of nearly 80 days. The study included adults and children aged 2 years and older, highlighting the potential of VTAMA cream to provide lasting control of AD symptoms without the need for continuous application.
“For patients with atopic dermatitis, the benefits of many topical treatments are often short-lived, and for some patients, their disease rapidly reappears after taking a break from topical treatments,” said Jonathan Silverberg, MD, PhD, MPH, of George Washington University School of Medicine and Health Sciences. “As a physician, I’m encouraged that at the end of a break from treatment with VTAMA cream, patients’ AD remained mild. I’m excited to see these data support the use of VTAMA cream to potentially provide lasting results for itch, which is the universal and most burdensome symptom of AD.”
Study design and results
ADORING 3 was a 48-week open-label, long-term extension study that enrolled 728 patients, including those who had participated in the pivotal Phase 3 ADORING 1 and ADORING 2 trials, as well as VTAMA cream-naive patients aged 2 to 17 years who did not meet the inclusion criteria for the previous studies. The study assessed the ability of VTAMA cream to maintain treatment success off-treatment.
Patients who entered the study with or achieved completely clear skin—defined as a validated Investigator Global Assessment for AD (vIGA-AD) score of 0—stopped using VTAMA cream. These patients were monitored to see if they could maintain a vIGA-AD score of 0 (clear) or 1 (almost clear) without treatment. If a patient’s AD returned to a vIGA-AD score of 2 or higher (mild or above), they were retreated with VTAMA cream until their skin cleared again or until the study concluded.
The results showed that at the end of the first treatment-free interval, which averaged 79.8 consecutive days, 84% of patients had a vIGA-AD score of 2, indicating mild disease. Additionally, the mean weekly Peak Pruritus Numerical Rating Scale (PP-NRS) score was 2.9, and the mean Eczema Area and Severity Index (EASI) score was 3.4, both suggesting mild disease activity.
Safety profile
The most frequent treatment-emergent adverse events (TEAEs) observed in the ADORING 3 study were folliculitis (12.1%), nasopharyngitis (6.9%), and upper respiratory tract infections (6.9%). The rate of discontinuation due to adverse events was low at 2.6%. Adverse events of special interest, including follicular events, contact dermatitis, and headache, were mostly mild or moderate, with low discontinuation rates of 1.0%, 0.4%, and 0%, respectively.
“These data reinforce the efficacy of VTAMA cream in atopic dermatitis, including the durability of effect among patients in the study, including children as young as two years old,” said Juan Camilo Arjona Ferreira, MD, head of research and development and chief medical officer at Organon. “Knowing the profound impact that atopic dermatitis can have on the lives of patients, and oftentimes their caregivers, the possibility to receive over two months of relief without needing to reapply treatment is meaningful and speaks to our mission of creating a healthier every day.”
Regulatory background
VTAMA cream was approved by the FDA in December 2024 for the topical treatment of atopic dermatitis in adults and children aged 2 years and older.2 This approval followed the FDA’s 2022 approval of VTAMA cream for the topical treatment of mild, moderate, and severe plaque psoriasis in adults.1
References
1. New Analysis of Organon’s VTAMA® (tapinarof) cream, 1% Phase 3 Data Shows Atopic Dermatitis Disease Activity Remained Low After Treatment-Free Interval in Adults and Children 2 Years of Age and Older. Organon. March 8, 2025. Accessed March 10, 2025. https://www.organon.com/news/new-analysis-of-organons-vtama-tapinarof-cream-1-phase-3-data-shows-atopic-dermatitis-disease-activity-remained-low-after-treatment-free-interval-in-adults-and-children-2-years-of-ag/
2. FDA Approves VTAMA® (tapinarof) cream, 1% for the Treatment of Atopic Dermatitis in Adults and Children 2 Years of Age and Older. Organon. December 16, 2024. Accessed March 10, 2025. https://www.organon.com/news/fda-approves-vtama-tapinarof-cream-1-for-the-treatment-of-atopic-dermatitis-in-adults-and-children-2-years-of-age-and-older/
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.