Tina Tan: Higher uptake in nirsevimab, maternal vaccine expected this RSV season
Contemporary Pediatrics' editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, talks RSV immunization and the availability of prevention tools.
In this video interview, Tina Tan, MD, FAAP, FIDSA, FPIDS, editor in chief of Contemporary Pediatrics, and professor of Pediatrics at the Feinberg School of Medicine, Northwestern University, highlighted why uptake for the monoclonal antibody nirsevimab (Beyfortus; Sanofi and AstraZeneca) could increase this season, as could Pfizer's Respiratory Syncytial Virus Vaccine (Abrysvo) uptake.
Pfizer's maternal vaccine was approved in August of 2023 as the first vaccine for use in pregnant individuals to prevent lower respiratory tract disease (LRTD) and severe LRTD as a result of respiratory syncytial virus (RSV) in infants, from birth through 6 months of age.1
Nirsevimab was approved by the FDA in July of 2023 to prevent RSV LRTD in and infants entering, or born during their first RSV season, as well as for children up to 24 months that remain vulnerable to severe RSV through their second season.2
Tan said partial reason for the low uptake last RSV season for nirsevimab was because of the documented limited supply availability after its approval in 2023. This RSV season, Tan said, adequate supply is expected for the monoclonal antibody.3
"Because there was a shortage of the nirsevimab, there wasn't quite as much uptake as could have occurred if there wasn't a shortage in the 100 mg dosage," said Tan. "But it's expected that this year, nirsevimab dosing will be adequate, and therefore there should be much better uptake of the nirsevimab."
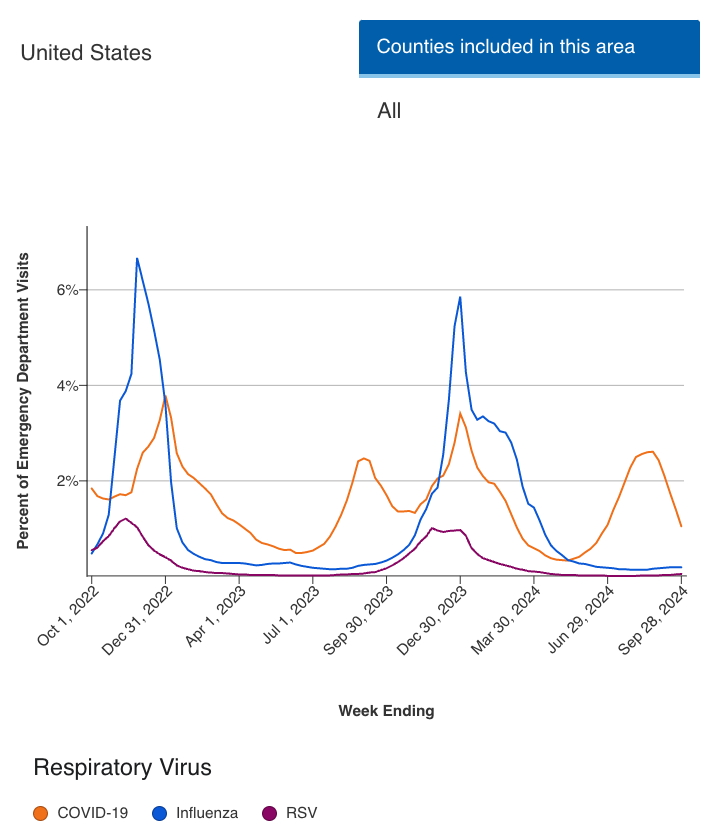
According to respiratory virus activity levels reported by the Centers for Disease Control and Prevention (CDC), overall acute respiratory illness causing individuals to seek health care is low.4
"Nationally, influenza and RSV test positivity are low. COVID-19 test positivity has decreased to 9.2%," the CDC lists on its website as of October 4, 2024.4
For the week ending of September 28, 2024, 0.1% of emergency department visits in the United States have been the result of RSV infection.4
Image credit: CDC.gov
"I think as we get better uptake of the monoclonal antibody and the vaccine, you're going to see a decrease in the amount of RSV hospitalizations and severe RSV cases," said Tan.
Nirsevimab data reported in The Lancet in May 2024 demonstrated that the monoclonal antibody reduced hospitalizations by 82% in infants under 6 months of age compared to those that received no RSV intervention.5
In all, 9408 infants in the seasonal and catch-up group received nirsevimab, while 348 infants received the monoclonal antibody in the high-risk group. Because there were "too few events in the high-risk group," according to study authors, only infants in the seasonal and catch-up groups were included in the analyses.5
References:
1. Fitch, J. FDA approves Pfizer’s maternal vaccine to prevent RSV in infants. Contemporary Pediatrics. August 21, 2024. Accessed October 9, 2024. https://www.contemporarypediatrics.com/view/fda-approves-pfizer-s-maternal-vaccine-to-prevent-rsv-in-infants
2. Fitch, J. Nirsevimab-alip FDA approved to prevent RSV in neonates, infants. Contemporary Pediatrics. July, 17, 2023. Accessed October 9, 2024. https://www.contemporarypediatrics.com/view/nirsevimab-alip-fda-approved-to-prevent-rsv-in-neonates-infants
3. Fitch, J. CDC recommends nirsevimab be prioritized for highest-risk infants amid limited availability. Contemporary Pediatrics. October 26, 2023. Accessed October 9, 2024. https://www.contemporarypediatrics.com/view/cdc-recommends-nirsevimab-be-prioritized-for-highest-risk-infants-amid-limited-availability
4. Respiratory Virus Activity Levels. CDC. Updated October 4, 2024. Accessed October 9, 2024. https://www.cdc.gov/respiratory-viruses/data/activity-levels.html
5. Fitch, J. Nirsevimab reduced RSV hospitalizations in infants by 82%, new data show. Contemporary Pediatrics. May 2, 2024. Accessed October 9, 2024. https://www.contemporarypediatrics.com/view/nirsevimab-reduced-rsv-hospitalizations-in-infants-by-82-new-data-shows
The Role of the Healthcare Provider Community in Increasing Public Awareness of RSV in All Infants
April 2nd 2022Scott Kober sits down with Dr. Joseph Domachowske, Professor of Pediatrics, Professor of Microbiology and Immunology, and Director of the Global Maternal-Child and Pediatric Health Program at the SUNY Upstate Medical University.