What you need to know about pediatric asthma pharmacology
With an array of asthma medications from which to choose, pediatricians must understand the pharmacology of the different drugs in order to make appropriate choices for each child. The author reviews the medications used for long-term asthma management and quick relief, delivery systems for these agents, and promising new drug therapies that lie on the horizon.
What you need to know about pediatric asthma pharmacology
By Nelson L. Turcios, MD
With an array of asthma medications from which to choose, pediatricians must understand the pharmacology of the different drugs in order to make appropriate choices for each child. The author reviews the medications used for long-term asthma management and quick relief, delivery systems for these agents, and promising new drug therapies that lie on the horizon.
Once a pediatric patient has been diagnosed with asthma, a treatment plan tailored to that child's particular needs has to be designed. In general, the goals of that plan are to eliminate and prevent symptoms, maximize pulmonary function, optimize pharmacotherapy while minimizing side effects, and allow the child to lead a normal life with as little disruption from the disease as possible.
To achieve these objectives, attention should be directed to four essential areas: Education of the patient, parents, and other caregivers; environmental control measures; regular assessment and monitoring; and pharmacologic therapy.1 The last item remains the basis of asthma management. Drug therapy must not only relieve acute asthma episodes but also prevent attacks, particularly in children with persistent symptoms. In this article I will discuss in detail the armamentarium of asthma medications available today, provide an overview of aerosol delivery systems, and give you a glimpse of potentially promising new therapies for managing asthma.
Long-term control medications
Asthma drugs can be divided into two main groups: medications used for long-term management of the disease and those used to obtain quick relief. Inhaled corticosteroids, cromolyn/nedocromil, leukotriene modifiers, long-acting ß2-agonists, and methylxanthines fall into the former category. Table 1 lists dosage forms and doses for these drugs.
TABLE 1
Long-term control medications
Inhaled corticosteroids remain the most effective therapy for patients with asthma because of their anti-inflammatory actions. With the new understanding that airway inflammation is present even in patients with mild asthma, inhaled steroid therapy is now recommended at a much earlier disease stage than in the past.
Inhaled corticosteroids quickly enter airway cells, where they bind to cytosolic receptors. These corticosteroid-receptor complexes move rapidly into the nucleus. There they bind to the corticosteroid-responsive elements of genes, either increasing or decreasing gene transcription. The most important action of corticosteroids is inhibiting transcription of several cytokines involved in airway inflammation, including interleukin (IL), IL-4, IL-5, and tumor necrosis factor-alpha (TNF-
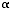
).2
Inhaled corticosteroids may also have direct inhibitory actions on many of the cells involved in asthmatic inflammationincluding eosinophils, T-lymphocytes, macrophages, and airway epithelial cells2and thereby produce anti-inflammatory effects on the bronchial mucosa. The drugs may not inhibit the release of chemical mediators from mast cells, but they do reduce the number of mast cells within the airway. Inhaled corticosteroids may also reduce microvascular permeability and mucus hypersecretion in inflamed airways.2 Among their several other actions, inhaled corticosteroids increase the synthesis of secretory leukocyte protease inhibitor (SLPI) by airway epithelial cells. SLPI is thought to be the predominant antiprotease in conducting airways and may be important in reducing airway inflammation.2 These drugs also induce production of ß2-receptors, making the action of ß2-agonists more effective.
By decreasing airway inflammation, inhaled corticosteroids consistently reduce airway hyperresponsiveness in patients with asthma.2 Long-term treatment diminishes airway reactivity to histamine, methacholine, and cold air, and decreases both early and late response to allergens.
Used regularly, inhaled steroids reduce the severity of exercise-induced bronchoconstriction (EIB). However, as many as 50% of patients who are well-controlled with inhaled corticosteroids still exhibit EIB. These drugs do not provide any protection against exercise-induced asthma when given immediately before exercise.
It may take one to three months for inhaled corticosteroids to lower the number of inflammatory cells in the airways, decrease shedding of airway epithelial cells, and reduce hyperplasia of epithelial goblet cells.2 When therapy is discontinued, symptoms and airway reactivity usually return to pretreatment levels.
Most inhaled corticosteroid preparations can be delivered by metered dose inhalers (MDIs) (see "A primer on aerosol delivery systems," below). Several dry powder inhalers (DPIs) are now on the market as well, and mometasone furoate with dry powder delivery is expected to enter the market soon in the United States. Budesonide inhalation for nebulization via compressed air-driven jet nebulizers has recently become available in the US. Its profile includes low mineralocorticoid activity and high anti-inflammatory action.
The pharmacokinetics of inhaled corticosteroids determine the proportion of drug that reaches target cells in the airways, as well as the fraction of each dose that enters the systemic circulation and causes side effects. After inhalation from a conventional MDI, a large proportion of the inhaled dose (80% to 90%) is deposited in the oropharynx and swallowed. This dose is then available for absorption into the systemic circulation after first pass liver metabolism. Between 10% and 20% of the inhaled dose enters the lower respiratory tract, where it is deposited in the airways and is then available for absorption into the systemic circulation. Newer breath-activated MDIs are said to deliver a greater amount of drug to the lower airways. Beclomethasone dipropionate is metabolized to monopropionate, an active compound. Budesonide and fluticasone, two newer agents, are metabolized to inactive compounds.
Local side effects caused by inhaled corticosteroids are associated with the dose, frequency of administration, and delivery system. Dysphonia (hoarseness) may develop in up to 30% of treated patients. It is probably the result of myopathy of the adductor of the vocal cords and is reversible when treatment is discontinued. Oropharyngeal candidiasis may be a problem for some children, but severe infection is rare. Cough and throat irritation, sometimes associated with reflex bronchoconstriction, may occur when inhaled corticosteroids are administered with a metered dose inhaler.
Systemic side effects caused by inhaled corticosteroids are a matter of concern, especially since these drugs are likely to be used for long periods and by children. The effect of inhaled steroids on linear growth is a particular concern. Controlled clinical studies have shown that inhaled corticosteroids, specifically beclomethasone dipropionate, may cause a reduction in growth velocity in pediatric patients. In these studies, the mean reduction in growth velocity was approximately one centimeter per year and appeared to be related to dose and duration of exposure. There have been fewer reports on budesonide and fluticasone, drugs characterized by high topical potency, rapid clearance once absorbed, low oral bioavailability, high affinity for corticosteroid receptors, and relatively inactive metabolites. One recent report showed that the effects of budesonide are limited to a small, transient reduction in growth velocity.8
For practical clinical application, it is important to recognize the following points:
- There are no studies that compare growth effects of therapeutically equivalent doses of inhaled corticosteroids. Most of the studies differ in the dose, delivery system, and study population.
- Inhaled corticosteroids produce considerably less growth suppression than oral corticosteroids and, in some instances, may produce the same clinical outcome. Inhaled corticosteroids vary in potency, delivery, and metabolism. These factors may influence the rate of growth, but not the final adult height.9
- A number of treatment approaches can assure the safe and effective use of inhaled corticosteroids in children. Monitor growth as frequently as possibleideally every two or three monthsusing a stadiometer. Prescribe the minimal effective dose and minimize the dosing frequency. Encourage patients to use spacer/holding chamber devices with MDIs and to rinse their mouth after inhalation to decrease local side effects and systemic absorption from the gastrointestinal tract. Use appropriate "add-on" medications and "steroid-sparing" strategiesfor example, environmental control and recognition and treatment of aggravating conditions such as rhinitis, sinusitis, and gastroesophageal reflux.
Oral corticosteroids are rarely needed in the long-term management of asthmatic children. In cases where they are necessary, prescribe the smallest amount that will control symptoms, to be taken as a single, alternate morning dose. Even with this approach, however, systemic side effects still may occur.
Cromolyn sodium/nedocromil sodium. Although cromolyn and nedocromil are chemically unrelated, they share a similar clinical profile. Both drugs are inhaled anti-inflammatory agents. While published results on the long-term effect of these drugs on airway reactivity have been inconsistent, cromolyn and nedocromil are much less effective in this respect than inhaled corticosteroids.10
Cromolyn works at the surface of the mast cell to inhibit its degranulation. This, in turn, prevents the release of histamine and inflammatory leukotrienes. The exact mechanism by which nedocromil exerts its anti-inflammatory effects is unknown. However, by interfering with mast cell degranulation, nedocromil inhibits the release of chemical mediators.
Although an individual's response to cromolyn is uncertain, the drug appears to be effective in 60% to 70% of children whose asthma is not controlled by bronchodilators. A four- to six-week trial of the drug given four times daily may be justified to determine its effectiveness, particularly in infants and very young children. Cromolyn seems to have little value in children who are dependent on steroids.
Cromolyn and nedocromil have been shown to effectively prevent symptoms triggered by anticipated exposures (allergens, exercise, cold air) on an as-needed basis. They may also reduce the need for quick-relief medications. One possible disadvantage of nedocromil is that it tastes bitter to some children. Both drugs have good safety profiles.
Leukotriene modifiers. The chemical structures of the material previously known as slow-reacting substance of anaphylaxis (SRS-A) are now called leukotrienes C4, D4, and E4. These molecules were so-named because the source molecule was originally isolated from leukocytes, and its carbon support contained three double bonds in series, which constitutes a triene. Leukotrienes mediate airflow obstruction, bronchial hyperactivity, and airway inflammation through multiple mechanisms. They exert their biologic action by binding and activating specific receptors.
Leukotriene modifiers include two groups of drugs: inhibitors of leukotriene synthesis (of which the drug zileuton is the only member) and leukotriene-receptor antagonists (Figure 1). Zileuton inhibits leukotriene synthesis by interfering with the catalytic action of 5-lipoxygenase, the enzyme that transforms arachidonic acid into the leukotrienes.11
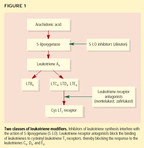
Most of the actions of leukotrienesincluding contraction of bronchial smooth muscle, eosinophil recruitment, increased mucus production, and increased vascular permeabilityare mediated by cysteinyl leukotriene T1 receptors. Leukotriene receptor antagonists block the binding of leukotrienes to these receptors. They carry the generic suffix-lukast: montelukast, zafirlukast, and pranlukast. (The latter is available only in Japan.)
In general, these medications have demonstrated their efficacy in preventing asthmatic responses to three important asthma precipitants: exercise, allergen exposure, and aspirin. One study reported that once-daily montelukast inhibited exercise-induced bronchoconstriction in 6- to 14-year-olds with asthma.12 Another study demonstrated that montelukast was even superior to salmeterol in preventing exercise-induced asthma.13 Such findings suggest a particularly important role for leukotriene antagonists, since children have an unpredictable schedule of physical activities.
Leukotriene antagonists appear to have additive effects when used concomitantly with inhaled steroids and may therefore improve asthma control with lower steroid doses. A recently published study reports that montelukast provided additional asthma control to patients benefiting from, but incompletely controlled on, inhaled beclomethasone.14 Preliminary data suggest that leukotriene antagonists also may have significant effects on nasal symptoms in patients with allergic rhinitis.15 Patients with asthma complicated by allergic rhinitis may require reduced nasal therapy if they receive leukotriene antagonists.
Leukotriene antagonists are indicated as first-line therapy for children with mild, persistent asthma and as adjunct therapy for those with moderate, persistent asthma who have a significant exercise-induced asthma component. Unfortunately, not all patients respond to these agents, and there is no method to determine clinically who will respond. The pediatrician needs to set goals and, after a six-to-eight-week trial, decide if treatment has been beneficial.
One advantage of these drugs is their simplicity of use. Leukotriene antagonists are oral agents that are dosed either once daily (montelukast) or twice daily (zafirlukast). Safety data indicate that their side-effect profiles are quite benign. In comparison with other asthma agents, their safety is matched only by cromolyn, and their ratio of efficacy-to-toxicity is excellent. In rare cases, adult patients have presented with clinical features of systemic eosinophilia, pulmonary infiltrates, cardiomyopathy, and other signs of vasculitis, consistent with Churg-Strauss syndrome.16 These events have usually been associated with a reduction in oral corticosteroid therapy while treatment with a leukotriene modifier was being initiated. No causal relationship has been established.
Zafirlukast is metabolized by cytochrome P450 and may interact with drugs metabolized in the same pathway. Infrequent adverse effects include mild headache, gastrointestinal disturbances, and increased liver function tests. Elevations in liver function tests are also seen in some patients taking zileuton, and it is necessary to periodically monitor liver function in patients taking this drug.
Long-acting ß2-agonists. Recent guidelines from the National Institutes of Health support the use of long acting ß2-agonists for chronic treatment of moderately severe asthma and prevention of exercise-induced bronchoconstriction and nocturnal asthma.1 A single long-acting ß2-agonist (salmeterol) is approved for use in the US, with a second long-acting ß2-agonist (formoterol) available in Europe and undergoing clinical evaluation in the US. Both drugs selectively activate the ß2-adrenergic receptor, causing relaxation of airway smooth muscle and thereby relieving and preventing bronchospasm. In contrast to the short-acting ß2-agonist albuterol, both salmeterol and formoterol produce clinically useful bronchodilation lasting about nine to 12 hours in most patients. Their duration appears to wane over the course of daily therapy.17 Sustained-release albuterol every 12 hours may be beneficial in children older than 12 years unable to use MDIs and for nocturnal asthma. However, it produces more adverse effects and has a slower onset of action than the same drug given by inhalation.
Salmeterol and formoterol do exhibit biophysical and functional differences.18 Formoterol has an onset of action within two to three minutes, whereas salmeterol takes effect in approximately 17 minutes. Current recommendations for salmeterol state that it should never be used in acute asthma or in place of anti-inflammatory medications. At present, we do not have enough information to know whether it is safe to use a long-acting ß2-agonist with a rapid onset of action, such as formoterol, to treat acute asthma symptoms.
Notwithstanding the concern about the regular use of ß2-agonists, the practitioner has to decide on the role of a long-acting ß2-agonist. Recent studies have demonstrated that the combination of a low-dose inhaled corticosteroid and a long-acting ß2-agonist is more effective than a high-dose inhaled corticosteroid alone.19 This observation holds true for different long-acting ß2-agonists (salmeterol and formoterol) as well as for different inhaled corticosteroids preparations (fluticasone and budesonide).
The mechanism by which long-acting ß2-agonists potentiate the activity of inhaled corticosteroids is unknown. One potential mechanism is suggested by in vitro studies evaluating corticosteroid receptor function in the presence of a long-acting ß2-agonist (salmeterol) with and without the corticosteroid (fluticasone). As noted above, corticosteroids exert their biologic function by binding to corticosteroid receptors in the cytoplasm of most cells. Once the corticosteroid binds to its cytoplasm receptor, that receptor translocates to the nucleus, where it exerts its biologic effect by activating approximately 10 to 100 genes.
In contrast to the cytoplasmic location of the corticosteroid receptor, the ß2-adrenergic receptor is a cell surface receptor that does not translocate to the nucleus when engaged by a ligand, such as salmeterol.20 Interestingly, salmeterol engagement of the ß2-adrenergic receptor induces translocation of a small amount of corticosteroid to the nucleus, but that amount is much less than that induced by the corticosteroid fluticasone. The combination of salmeterol and fluticasone, however, is much more effective in inducing translocation of the corticosteroid receptor from the cytoplasm to the nucleus than either drug alone. A preparation combining fluticasone and salmeterol has just become available in the US.
Another concern about long-acting ß2-agonists is that tolerance may develop with long-term use, resulting in a lack of patient response to short-acting ß2-agonists. To date, this concern has not been substantiated.
Methylxanthines. Compared with ß2-agonists, the onset of action of theophylline is slower and its peak bronchodilator effect lower. Theophylline has become a minor player in asthma management. The trend now is toward introducing this drug later in the treatment plan, for several reasons:
- Long-term use does not appear to cause any sustained improvement in airway hyperreactivity in asthma
- Patient satisfaction is low because of significant side effects, cognitive, behavioral, and learning difficulties experienced by some children, and the need to monitor blood concentrations
- Theophylline increases the risk of mortality from asthma.
Current recommendations include the use of theophylline as a long-term controller in moderate to severe asthma and in children with nocturnal asthma. Its use can decrease inhaled corticosteroid requirements.21 If you prescribe theophylline choose a sustained-release preparation and adjust the dosage to serum theophylline levels of 12 to 15 mg/L. As a precaution, advise parents to reduce the dose by 50% if their child has a prolonged fever and not to administer any other medication without first establishing the safety of the combination. Concurrent use of zafirlukast and theophylline can decrease the effects of zafirlukast. Zileuton can decrease theophylline clearance and markedly increase theophylline serum concentrations.
Quick-relief medications
This group of drugs provides prompt relief of bronchoconstriction and associated acute symptomscoughing, wheezing, shortness of breath or rapid breathing, and chest tightness. It includes short-acting ß2-agonists, oral corticosteroids, and anticholinergics. Table 2 lists dosage forms and doses for these drugs.
TABLE 2
Quick-control medications
Short-acting ß2-agonists. ß2-agonists are the most potent bronchodilators available.22 Short-acting ß2-agonists include albuterol, terbutaline, pirbuterol, bitolterol, and levalbuterol. They should be delivered by inhalation to ensure rapid onset of action and minimize adverse effects. When delivered by this route, they result in bronchodilation within five to 10 minutes and have a duration of action of four to six hours. They are the therapy of choice for relieving acute symptoms and preventing exercise-induced bronchospasm.
Albuterol, the most commonly used ß2-agonist, is a 50/50 racemic mixture of two mirror image isomers termed (R)-albuterol and (S)-albuterol. (R)-albuterol (levalbuterol) is responsible for the rapid bronchodilator effects of the racemic mixture, whereas (S)-albuterol has essentially no bronchodilator properties.23 The role of the inactive (S)-albuterol has recently been reexamined in view of controversies about the possible deleterious effects of regular use of short-acting ß2-agonists. Studies suggest that (S)-albuterol may increase airway reactivity and cause loss of asthma control.
The pharmacokinetics of (S)-albuterol differ from those of (R)-albuterol, with (S)-albuterol being metabolized 10 times more slowly. Regular repeated dosing of the racemic mixture of albuterol exposes the patient to more of the potential adverse effects of (S)-albuterol than it does to the potential beneficial effects of (R)-albuterol. A recent study involving moderate to severe asthmatics compared different doses of nebulized (R)-albuterol administered alone with equivalent amounts of nebulized (R)-albuterol administered as a racemic mixture.24 The results suggest that a 0.63mg (R)-albuterol dose may have a better therapeutic index than the 2.5 mg standard racemic albuterol dose. However, Asmus and colleagues recently reported that (R)-albuterol offers no clinically significant advantage over racemic albuterol.25
To sum up, some short-acting ß2-agonists have active as well as inactive ingredients that may have adverse effects. In addition, asthmatics vary in their clinical response to short-acting ß2-agonists, which probably reflects differences in ß2-receptor genotype.
Oral corticosteroids. Short courses (three to seven days) of oral corticosteroids are remarkably effective at gaining initial control of acute asthma and speeding resolution of moderate or severe persistent exacerbations. Although even short courses can cause adrenal suppression, adrenal function returns rapidly. Suppression may be prolonged, however, in children who receive more than four short courses of oral corticosteroids per year.
Anticholinergics. Ipratropium bromide, an atropine derivative, works by blocking the binding of acetylcholine to the M3 receptor, resulting in bronchodilation. Compared with ß2-agonists, it has a slower onset of action30 to 90 minutesbut a longer duration of action. It is currently available as an MDI and as a nebulizer solution.
Ipratropium may have an additive effect when used with inhaled albuterol in children with severe exacerbations. A study comparing the efficacy of ipratropium added to albuterol therapy in the emergency room found a significant reduction in the rate of hospitalization in the ipratropium/albuterol group compared with the albuterol only group.26
Although the cholinergic pathway contributes to the symptoms of acute asthma, its importance should not be overestimated. A meta-analysis reported the effect of ipratropium bromide on patients who were admitted and treated with ß2-agonists.27 None of the studies found these drugs to be beneficial in terms of clinical rating score, admission rate, or length of hospital stay, and researchers concluded that there was no support for their use in hospitalized children.
Ipratropium has a less clearly defined role in the long-term treatment of asthma. It can, however, be used as an alternative bronchodilator for children who do not tolerate inhaled ß2-agonists and those with psychogenic asthma. In this condition, a patient becomes psychologically stressed, the vagus nerve is activated, and acetylcholine is released in the lungs. For the most part, the role of ipratropium is limited to adults with chronic bronchitis. Dry mouth, pharyngeal irritation, urinary retention, and increases in intraocular pressure occur rarely with the use of ipratropium.
New treatment strategies
Researchers are investigating several new therapeutic approaches that may benefit patients with asthma.
Monoclonal anti-IgE antibody. Immune responses mediated by immunoglobulin E (IgE) are important in the pathogenesis of asthma. Recombinant humanized monoclonal antibody (rhuMAb-E25) forms complexes with unbound (free) IgE but not with IgG or IgA. It blocks the binding of IgE to cell-membrane receptors, thereby inhibiting the release of mediators, but it does not bind to cell-bound IgE.
Results of a double blind, placebo-controlled, clinical trial were recently published.28 The trial involved more than 300 patients between 11 and 50 years of age who had moderate to severe asthma. Patients were randomized to receive either intravenous placebo or low- or high-dose rhuMAb-E25 every two weeks. After 20 weeks of treatment, no patient had antibodies against rhuMAb-E25, and patients who received it showed greater clinical improvement than those who received placebo. These findings suggest that treatment with antiIgE antibody may be useful in patients with severe asthma who require oral corticosteroids.
Dissociated steroids. Dissociating anti-inflammatory effects of corticosteroids that are mediated by inhibition of transcription factors from side effects mediated by glucocorticoid receptors binding to DNA may lead to a new generation of "dissociated steroids." These drugs may retain anti-inflammatory efficacy with fewer systemic effects.29
Anti-cytokine antibodies. A complex network of cytokines are thought to be responsible for maintaining the chronic inflammation found in asthma. Cytokines that are especially important in the diseasesuch as IL-4, IL-5, IL-13, and TNF-
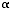
may be targeted nonspecifically by drugs that inhibit synthesis (corticosteroids or cyclosporin A) or by using blocking antibodies that are specific for cytokines or their receptors.30
The pharmacologic options for treating asthma in children will, we hope, continue to expand. In the meantime, successful management depends to a large extent on how well versed pediatricians are in the pharmacology of currently available agents and how well they apply that knowledge to the individual needs of their patients.
REFERENCES
1. National Asthma Education and Prevention Program: Expert Panel Report II. Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD, US Department of Health and Human Services. Publication No. 97-4051,1997
2. Barnes PJ: Inhaled glucocorticoids for asthma. N Engl J Med 1995;332:868
3. Dolovich M: Aerosol delivery to children: What to use, how to choose. Pediatr Pulmonol 1999;18:S79
4. O'Callaghan C: Delivery Systems: The Science. Pediatr Pulmonol 1997;15:551
5. Bailey WC, Gerald LB: Inhalers for patients with asthma: New perspectives J Respir Dis 1998;19(11):956
6. Wildhaber JH, Dore MD, Wilson JM, et al: Drug deposition comparable with nebulizer and MDI in pediatric asthma. J Pediatr 1999;135:28
7. Everard ML, Clark AR, Milner AD: Drug delivery from holding chambers with attached facemask. Arch Dis Child 1992;67:580
8. The Childhood Asthma Management Program Research Group: Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054
9. Silverstein MD, Yunginger JW, Reed CE, et al: Attained adult height after childhood asthma: Effect of glucocorticoid therapy. J Allergy Clin Immunol 1997;99:466
10. Krawiec ME, Wenzel SE: Inhaled nonsteroidal anti-inflammatory medications in the treatment of asthma. Respir Care Clin North Am 1999;5(4):555
11. Drazen JM, Israel E, O'Byrne PM: Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 1999;340:197
12. Kemp JP, Dockhorn RJ, Shapiro GG, et al: Montelukast once daily inhibits exercise-induced bronchoconstriction in 6- to 14-year-old children with asthma. J Pediatr 1998;133:424
13. Villaran C: Montelukast vs. salmeterol in patients with asthma and exercise-induced bronchoconstriction. J Allergy Clin Immunol 1999;104:547
14. Laviolette M, Malmstrom K, Lu S, et al: Montelukast added to inhaled beclomethasone in treatment of asthma. Am J Respir Crit Care Med 1999;160:1862
15. Spector S: Pathophysiology and pharmacology of allergic rhinitis. J Allergy Clin Immunol 1999;103(3pt2): S237
16. Wechsler ME, Pauwels R, Drazen JM: Leukotriene modifiers and Churg-Strauss syndrome: Adverse effect or response to corticosteroid withdrawal? Drug Saf 1999; 21(4)241
17. Lenney W, Pedersen S, Boner AL, et al: Efficacy and safety of salmeterol in childhood asthma. Eur J Ped 1995;154:983
18. Anderson G: Long-acting inhaled b adrenoreceptors agonists: The comparative pharmacology of formoterol and salmeterol. Agents Actions 1993;43:253
19. Pauwels RA, Lofdahl CG, Postma DS, et al: Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1998;337:1405
20. Rhodes D, Newton R, Butler R, et al: Equilibrium and kinetics of the interaction of salmeterol with membrane bylayers. Mol Pharmacol 1992;42:591
21. Evans DJ, Taylor DA, Zetterstrom O, et al: A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide in moderate asthma. N Engl J Med 1997;337:1412
22. Turcios NL: ß2-adrenergic agents in asthma. Pediatr Rev 1996;17:103
23. Penn RB, Frielle T, Mccullough JR, et al: Comparison of R-, S-, and RS-albuterol interaction with human b1 and ß2-adrenergic receptors. Clin Rev Allergy Immunol 1996;14:37
24. Nelson H, Bensch G, Pleskow, et al: Improved bronchodilation with levalbuterol compared with racemic albuterol in patients with asthma. J Allergy Clin Immunol 1998;102:943
25. Asmus MJ, Hendeles L: Levalbuterol nebulizer solution: Is it worth five times the cost of albuterol? Pharmacotherapy 2000;20(2)123
26. Schuh S, Johnson DW, Callahan S, et al: Efficacy of frequent nebulized ipratropium bromide added to frequent high-dose albuterol therapy in severe childhood asthma. J Pediatr 1995;126:639
27. Osmond MH, Klassen TP: Efficacy of ipratropium bromide in acute childhood asthma: A meta-analysis. Acad Emerg Med 1995;2:651
28. Milgrom H, Fick RB Jr, Su JQ, et al: Treatment of allergic asthma with monoclonal anti-IgE antibody: rhuMAb-E25 Study Group. N Engl J Med 1999;341:1966
29. Vayssiere BM, Dupont S, Choquart A, et al: Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit anti-inflammatory activity in vivo. Mol Endocrinol 1997;11:1245
30. Barnes PJ, Lim S: Inhibitory cytokines in asthma. Mol Medicine Today 1998;4:452
THE AUTHOR is Associate Professor of Pediatrics and Director of Pediatric Pulmonology/Cystic Fibrosis at the University of Medicine and Dentistry of New JerseyNew Jersey Medical School, Newark.
A primer on aerosol delivery systems
Asthma medications should be delivered by inhalation whenever possible. The rationale for using the inhaled route is that medication is deposited directly in the airways, resulting in a more rapid onset of action, effectiveness with much smaller doses, and fewer systemic side effects.3 To obtain full therapeutic effect, an adequate amount of the inhaled drug must be deposited in the lungs. The accuracy of delivery is limited by the size of the aerosol particle, the patient's breathing technique, and airway patency.
The particles that make up a given aerosol vary in size. The mass median aerodynamic diameter (MMAD), used to describe particle size distribution, is always such that 50% of the mass of the aerosol is contained within larger particles and 50% is contained within smaller particles. Particles smaller than 5 µm are deposited predominantly in the lung, whereas particles larger than 5 µm are deposited in the nasopharynx (or oropharynx with mouth breathing). Particles smaller than 5 µm are therefore defined as within the respirable range. Particles larger than 1 mm are deposited by inertial impaction in regions of the respiratory tract where airflow velocity is high and airflow changes direction rapidly. For this reason, inertial impaction of aerosol particles occurs predominantly in the nasopharynx (or oropharynx with mouth breathing) and at bronchial bifurcations. Particles larger than 0.5 µm are deposited in smaller airways by gravity-assisted sedimentation. Breath holding of up to 10 seconds enhances the residence time, increasing deposition. Particles smaller than 0.5 µm are deposited primarily by diffusion beyond the terminal bronchioles. Several devices are available for delivering aerosolized medications:
Metered dose inhalers deliver small particles of medication to narrow lower airways. MDIs are small, pressurized canisters that contain a drug either in suspension or solution, both of which require a propellant. The chlorofluorocarbon (CFC) propellants in these formulations are being replaced for environmental reasons, usually with hydrofluoroalkanes (HFAs). The velocity of the spray with HFA-MDIs is slower than with CFC-MDIs and the spray has a smaller particle size, so more of the drug may be delivered to the lower airways. Although MDIs appear simple to use, simultaneous coordination of inhalation and activation of the aerosolwhich is essential for effective drug deliverymay be difficult for as many as 50% of children.
Some MDIs are breath activated. These MDIs are still pressurized with CFCs, which provide the driving force. Aerosol is automatically released on inhalation at inspiratory flow rates as low as 30 L/min, thus avoiding activation/ inhalation difficulties. However, these devices require a relatively high inspiratory flow to generate a predictable dose of aerosol, are not suitable for children under about 5 years of age, and may not activate under conditions of extreme airflow obstruction.
Spacer devices (also known as holding chambers and extension tubes) are designed to overcome the problems associated with coordination of inhalation and aerosol release. Spacers decrease aerosol particle size by evaporation and impaction of large particles on the walls and valves of the device. This reduces oropharyngeal deposition 10- to 15-fold, thus decreasing total body dose. It also lowers the cost of aerosol therapy. Spacers with a volume in excess of approximately 140 mL usually increase the total dose delivered to the lower respiratory tract. Valved holding chambers virtually assure aerosol delivery to infants, children, and adults.
Spacer devices with face masks have proven useful in treating patients of all ages and are now the first choice for delivery of antiasthma aerosols to children 5 years of age or younger.4 Six normal inspirations are necessary for each activation of the inhaler when a spacer device with face mask is used. Only one normal breath is required with a spacer and mouthpiece. The inhaler should be activated only once while the patient is inhaling.
Studies have demonstrated that a delay between activation and inhalation can reduce the fine particle mass of drug available. A delay of 20 seconds, for example, can reduce the drug available for inhalation by up to 80%.
Dry powder inhalers. Unlike pressurized metered-dose inhalers, DPIs do not require propellants. They are activated and driven by patients' own rapid inspiratory force, so the user must breathe in vigorously and quickly to receive the correct dose of medication. Advantages include the fact that patients do not have to coordinate activation with inhalation for effective delivery, spacers are not necessary, and dosing can be monitored. Because DPIs are breath activated, they are not recommended for children less than 5 years of age. It appears easier to achieve good technique with minimal instruction using DPIs than it is with MDIs.5 Both single-dose and multiple-dose DPIs are available.
DPIs have two noteworthy disadvantages: Particles of drug may form nonrespirable agglomerates, resulting in poor penetration to lower airways, and inhalation of the powder itself may cause irritation.
Nebulizers. The main advantage of the nebulizer is that it requires little patient coordination. It therefore seems to be the preferred way to deliver aerosols to infants and small children and those with severe asthma. To ensure optimal lung deposition of drug particles, a gas flow rate of 68 L/min and dilution of the drug in a nebulizer to a volume of 3 mL is recommended.
There are two types of nebulizer devices: jet and ultrasonic. The jet nebulizer passes compressed air over a tube, one end of which rests in the liquid to be aerosolized. The pressure drop draws liquid up the tube, where it is broken into droplets of various sizes. Ultrasonic nebulizers generate particles by concentrating vibrations from a piezoelectric transducer on the surface of the liquid to be aerosolized. Of the dose of aerosol that is generated by either nebulizer, an average of 10% is deposited in the lungs. A metered dose inhaler or a valved spacer with a mask can accomplish the same goal more efficiently, however.6 Breath activation is becoming a design feature in newer nebulizers, and these devices should be available soon.
Technique is extremely important in nebulized therapy. For example, one study demonstrated that the dose inspired when the nebulizer mask is moved 1 cm from the child's face is only 50% of that inspired when the mask is in contact with the child's face, and a distance of 2 cm resulted in an 80% reduction.7 Additionally, the use of a mouthpiece whenever possible has been shown to increase the amount of drug delivered to the lungs. Hopefully, this information will discourage the commonly used "blow-by" technique.
Nelson Turcios. What you need to know about pediatric asthma pharmacology. Contemporary Pediatrics 2001;1:81.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.