Where are we at with COVID-19 vaccines for children?
A pediatric infectious disease specialist discusses where we are at with children's vaccines for COVID-19.
Sean O’Leary, MD, is a pediatric infectious disease specialist and an associate professor of pediatrics at the University of Colorado. Among his several partnerships with immunization and infectious disease organizations, he is a liaison to the Advisory Committee on Immunization Practices (ACIP) for the Pediatric Infectious Disease Society. I spoke with Dr O’Leary this week after his recent meeting with the ACIP to discuss children’s vaccines for coronavirus disease 2019 (COVID-19) and multisystem inflammatory syndrome in children (MIS-C).
Q: What can you tell us, in general, about what is happening with children’s trials for COVID-19?
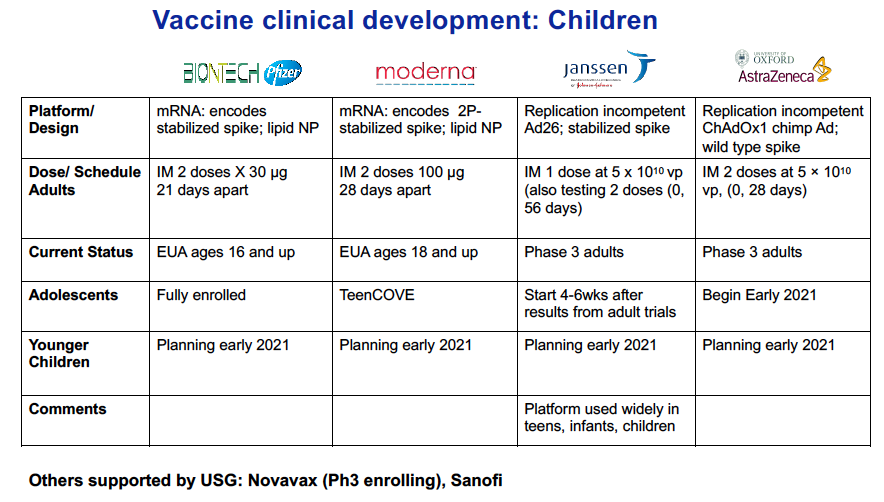
Well here is where we are: The Pfizer vaccine right now is licensed down to children aged 16 years; their new trial for adolescents aged 12-15 years is now fully enrolled. They are looking to submit that data to the FDA and then, possibly get authorization in the first half of this year.
Moderna is in the process of doing a study in children aged 12 to 17 years, and just beginning enrollment, getting their sites set up, that sort of thing. I am told that Janssen (owned by Johnson & Johnson) is in the planning stage for a similar trial.
Once you get down to the younger ages, those plans are starting to take shape, but no enrollment quite yet.
The trials are somewhat complicated because they’re probably not going to enroll nearly the numbers they did in the adult trial, which were at 30,000 to 45,000. What they are going to be looking for, is, are the vaccines safe in this population and do they induce a similar immune response to the age group that is right above them where we know its effective. So, for example, they will compare 12–15-year-olds, in, say, the case of the Pfizer product, to teens and young adults aged 16 to 24 years, something like that, to see if the immune response and safety profile looks similar. Then, knowing that it is efficacious in that older group, they can then presume efficacy in the younger age group, rather than powering the trial to the point we saw in the adult trials. The big picture around the pediatric clinical trials is these immune-bridging studies with age de-escalation. The age de-escalation mean going slowly down in age categories over the coming months. In some of the younger age categories, they are going to be looking at the right dose. So they may, in a certain age group, look at a full dose compared to a half-dose. And then, if the half-dose looks just as robust, then they might do a half dose versus a quarter dose, that sort of thing. And so those trials are being planned right now.
Q: So it sounds like the trials will consist of using the current vaccines that have come to market?
Yes. And in terms of where the trials are at: For Pfizer, since they have already finished their enrollment down to age 12, are planning children aged 5 to 11 years next. And then, Moderna have enrolled a few but trying to get their sites in place, talking to a lot of different sites around the country. The FDA really prefers to approve vaccines based on trials performed in the United States.
Q: Any indication when the trials will actually start, when everything is in place?
Well, for Moderna, very soon, in the coming days to weeks. I am not sure about Johnson & Johnson. I have read that they are enrolling in other countries, will not participating with the entity formally known as Operation Warp Speed but planning them on their own.
Q: Do you think the trials will be as quick as the adult trials have been?
These companies are scaling up their manufacturing capacity as quickly as they can. The Pfizer product is already out there, it’s same dose down to age 12; and so, presumably children can get that, it is just a matter of supply. The hope is that once kids are in the queue, so to speak, they will then have an adequate supply. A lot of this will depend on these other vaccines. So, the Pfizer product is the one most likely to be available to children first. And, once they open up the high-risk categories, those teens that are high risk could be vaccinated as well. We also have to think about other phases, so if the Johnson and AstraZeneca products get an EUA from the FDA in the coming months, that will greatly increase our supply across the United States. Therefore, the Pfizer product may be able to be diverted to children. On the other hand, if we have really constrained supplies come May, June, then that will be an issue because we will still need to go through other phases, essential workers, high risk conditions, etc.
Q: What are the differences in using a vaccine for children compared to adults?
I get that question a lot. There are a few examples out there where there are some differences in immunogenicity or reactogenicity based on age. The dengue vaccine for example, that we don’t use in the United States, but is licensed by the Food and Drug Administration (FDA), is a tricky vaccine because in certain kids who haven’t had dengue before, it may actually lead to a more severe disease. So that’s an example of where it needed to be tested in children because there is a difference. We always like to have trials done in kids because, as pediatricians, if we are going to talking with parents about vaccinating their children, we want to be able to point to the data and say, these were tested and were shown to be safe and effective in children, as opposed to just assuming they are because the adults have them. We hold vaccines to a much higher safety standard than any other medicine we give, because we are giving them to a healthy population and so we do want to have them tested in kids.
The other wrinkle with these vaccines compared to other ones is (MIS-C). That seems to be an immune phenomenon, we don’t fully understand the path or physiology of it. So if that’s the case, we are giving a vaccine to induce a response, could the vaccine trigger MIS-C? Most people think probably not. That the path of physiology of MIS-C is probably more complex than simply responding to the spike protein, but we don’t really know that. That’s another reason we really want to make sure these vaccines are shown to be safe in children. Some of these vaccines may be given to children in other countries. At the ACIP meeting, it was asked if there was any reaction at all of MIS-C presenting in younger adults (20-year-olds) in the ongoing trials. The head of the immunization and safety office at the Centers for Disease Control and Prevention had not heard of any at this point. So that is somewhat assuring.
Q: Will children who tested positive for COVID-19 be able to be part of a vaccine trial?
Probably not. For the Pfizer recruitment, you couldn’t have had a known history of COVID. So a known history of symptomatic COVID or MIS-C will most likely be exclusionary criteria for these other pediatric trials. The obvious reason is you want to be able to show efficacy and if you have people that have been previously or recently infected and may have some immunity already, it would be bias toward null. It can water down your study population, so you can’t tell as well if the vaccine works.
Q: Any thoughts about all these new strains being reported? Are children more vulnerable to them?
We simply don’t know yet. It remains to be seen, and they are being watched closely. What I have read and seen is yes, they may be more contagious. All the more reason why we must continue our physical distancing measures, wear masks when out, avoid large gatherings, doing all we can to prevent the spread. Those measures still should work with new strains. The other piece is schools, and we have to watch that closely. I have been strongly advocating for children to be back in schools, and what we have seen, as you know, is with safety measures in place, transmission in schools is very, very low. But we need to watch it closely with these new variants.
Q: And finally, what are your thoughts/concerns on vaccine hesitancy?
Those (are issues) we will need to deal with families regarding that for sure. I am hearing from pediatricians all over the place that families are bringing this up in conversations, vaccines for their kids. Some want to vaccinate their children as soon as possible, because of a preexisting condition, others are skeptical. We still have a lot of work to do in that arena for sure. The American Academy of Pediatrics, as you know, has been advocating strongly to move these clinical trials in children along as quickly as possible. Both because of recognizing the need to vaccinate the 74 million children in this country to achieve herd immunity, but also to protect them as well. It’s not right to say it’s a benign disease in children. It’s less severe, but we still need to protect children from it.