Acetaminophen: The other side of the story
This widely used OTC drug has minimal side effects, but it's easier than parents realize to give a child too much. Find out how these therapeutic misadventures come about and what you can do to prevent them.
Acetaminophen: The other side of the story
By James E. Heubi, MD
This widely used OTC drug has minimal side effects, but it's easier than parents realize to give a child too much. Find out how these therapeutic misadventures come about and what you can do to prevent them.
Acetaminophen has been available as a nonprescription drug since 1960 and--despite some debate--remains the drug of choice for reducing fever in children. Acetaminophen has a long history of safe use with few reported serious adverse effects from the dosages recommended by the manufacturers. A recently published study evaluating 28,130 children exposed to acetaminophen failed to show any increased risk of acute gastrointestinal bleeding, acute renal failure, anaphylaxis, or Reye syndrome.1 Although minor adverse effects may not have been noted in this research, authorities agree that the side effects of this drug are minimal.
Acetaminophen use has increased substantially in the United States since the mid-1980s, when epidemiologic evidence incriminated aspirin in the development of Reye syndrome. Today, most children receive acetaminophen, ibuprofen, or a combination of the two for the treatment of fever. Despite its recognized safety in appropriate therapeutic doses, there are at least three circumstances in which acetaminophen may be toxic:
- Intentional overdose, with single doses exceeding 140 mg/kg
- Unintentional overdose in which a child is given multiple doses of acetaminophen that exceed those recommended by the manufacturer
- Inadvertent overdose in which a child is given both acetaminophen for fever and a cough-and-cold preparation containing acetaminophen.
The first of these possibilities is a less serious concern than it used to be. While it is true that deliberate overdoses of acetaminophen can lead to severe hepatic necrosis and hepatic failure, an effective antidote is now available. When administered up to 24 hours after a single toxic dose of acetaminophen, N-acetylcysteine (Mucomyst) can prevent serious toxicity.2,3 Since the introduction of this drug in the mid-1970s, mortality from intentional overdose has fallen to 0.43%, with rare deaths occurring when treatment is delayed.
My focus here will be so-called therapeutic misadventures. Pediatricians can help reduce their frequency and severity if they know how these accidental overdoses come about, how to recognize and treat them, and what to tell parents about prevention.
How big is the problem?
Despite millions of doses of acetaminophen being administered to infants and children over the last four decades, few cases of hepatocellular necrosis caused by multiple excessive doses have been reported in the medical literature. Recent reports of hepatotoxicity in chronic alcoholics taking therapeutic doses of acetaminophen drew attention to the potential problem,4 and two recent examinations of large numbers of children have demonstrated its scope.57
One study described a 10-year experience in five California-based tertiary care centers (four of them transplant centers) with intentional and unintentional acetaminophen overdoses in children less than 19 years of age. Sixty-two of the 63 suicidal patients in the study who overdosed survived, with three of them requiring orthotopic liver transplant. The single death in the suicide group was a patient who was also receiving isoniazid, which may have enhanced the toxicity of acetaminophen. Of the 10 children in the study whose overdoses were accidental, nine had significant hepatotoxicity and one died. The affected children had received from 75 to more than 750 mg/kg/day of acetaminophen, for periods of one to five days. These amounts, in all cases, exceed the doses given in the labeling instructions of 10 to 15 mg/kg/dose, not to exceed five doses/day. On the basis of this study, the estimated therapeutic index for acetaminophen is as low as 1.7. (The therapeutic index is calculated by dividing toxic dose by therapeutic dose.)
The incorrect doses given to these children occurred when teaspoonful quantities of infant drops (80 mg/0.8 mL) were given in place of liquid (160 mg/5 mL) and when regular strength tablets (325 mg) were substituted for chewable children's tablets (160 mg).
The second report, which my colleagues and I published in 1998, is a compilation of all previously published studies, cases reported to the Food and Drug Administration, and cases from the Children's Hospital Medical Center in Cincinnati. From these sources, we identified 47 children who had received unintentional overdoses of acetaminophen. They ranged in age from 5 weeks to 10 years and had received between 60 and 420 mg/kg/day of acetaminophen over a period of one to 42 days. Twenty-four of 43 (55%) died; five people received liver transplants, and four survived. Nine patients received N-acetylcysteine, and four of these children survived. Serum acetaminophen levels were available in 30 patients for whom the last dose could be determined with certainty, and 22 of these 30 (73%) had levels greater than the toxic range described for acute ingestion.
How did these overdoses come about? Twenty-three of the patients (52%) were given adult preparations (325 or 500 mg tablets or caplets), including seven of 21 children age 2 or younger and 16 of 23 children older than 2 years. An additional small number of children less than 2 years old were given infant drops at dosages recommended for the less concentrated liquid preparations.
Taking these two studies together, there have now been reports of more than 55 cases of severe hepatotoxicity associated with accidental overdoses of acetaminophen. This number is small when compared to the total number of children taking acetaminophen, but it is likely to be a gross underestimate of the total number of cases. My personal observation, based on conversations with physicians at liver transplant centers, is that doctors often see liver failure associated with multiple excess doses of acetaminophen, though most cases are not reported in the literature or to the FDA, and that many less severely affected patients go unreported as well.
What's the typical scenario?
Usually, the story begins when a child has a respiratory illness with fever. As indicated in the studies just described, a caregiver may give a young child multiple too-high doses of infant drops or liquid, or substitute infant drops for the less concentrated liquid preparations. With an older child, the caregiver may substitute adult tablets for the chewable tablets marketed for children. Or the adult may administer both antipyretic doses of acetaminophen and doses of cough-and-cold preparations that contain acetaminophen; the total amount of acetaminophen ingested may then reach toxic levels.
The parent may consult the pediatrician when, after multiple overdoses, the infant or child is nauseated, vomits, and becomes lethargic. Unfortunately, by the time these symptoms appear, significant liver injury may already have occurred, as indicated by markedly elevated serum liver enzymes (ALT, AST), prolonged prothrombin time, and elevated serum ammonia. Typically, the ALT and AST elevations reflect severe centrilobular necrosis. In one series, peak serum AST ranged from 1,180 to 30,000 IU/L and ALT ranged from 440 to more than 22,000 IU/L.7 As shown in Figure 1, peak serum AST and ALT values in infants and children with acetaminophen-induced liver injury are strikingly higher than peak AST and ALT in children who have fulminant hepatic failure from other causes.

When children with acetaminophen toxicity arrive at the hospital, they may be jaundiced and suffering from encephalopathy and coagulopathy. Renal injury and insufficiency are common, either as a direct result of toxicity or as a consequence of liver failure (hepatorenal syndrome).
The only treatment for children in this state is supportive care, and the mortality rate is high. Support should include treatment of hyperammonemia with lactulose or neomycin, attempts at correction of coagulopathy with vitamin K, careful fluid management with adequate provision of glucose to prevent hypoglycemia, and correction of electrolyte disturbances. Early transfer to a center equipped to perform pediatric liver transplantation should be arranged. Because they were in good health until the acetaminophen-induced liver failure, these patients are excellent candidates for transplantation.
The use of N-acetylcysteine may be beneficial for some, since its antioxidant properties may promote recovery of the massively injured liver. Circumstantial evidence suggests that N-acetylcysteine may benefit patients even when more than 24 hours has elapsed since their last exposure to acetaminophen. The toxicity from N-acetylcysteine appears trivial.
How acetaminophen damages the liver
In therapeutic doses, only a small amount of the acetaminophen in the dose requires conjugation with glutathione. After a toxic dose, however, glutathione stores can become depleted, as shown in Figure 2. Although it was long believed that a 70% depletion of glutathione was required before toxicity could occur, it is now evident that smaller depletion can be associated with toxicity.8,9 The area of injury is Zone 3 or the centrilobular portion of the hepatic lobule, with injury extending to Zones 1 and 2 in very severe cases, leading ultimately to collapse of the lobule and liver failure.

With repeated overdoses, changes in the metabolic pathways may affect the development of hepatic injury. In most multiple accidental overdoses, infants and children are febrile and acutely malnourished as a result of their illness. Reductions in caloric or protein intake combined with multiple doses of acetaminophen may lead to even greater reductions in hepatic glutathione, increases in turnover, and reductions in synthesis than would occur with acetaminophen alone.10 There is also a trend toward increased serum acetaminophen levels in children with repeated dosing; the younger the child, the larger the increase in serum acetaminophen.11 Polymorphisms in expression of the P450 enzyme may make some children more susceptible to liver injury.12 Certain drugs such as phenobarbital, phenytoin, rifampin, ethanol, and isoniazid may stimulate the P450 enzyme system and lead to enhanced production of N-acetyl-p-benzoquinone imine (NAPQI) and toxic metabolites. These alterations, singly or in combination, potentiate acetaminophen toxicity after multiple accidental overdoses.
How can overdoses be prevented?
Acetaminophen was first marketed by McNeil Consumer Healthcare under the brand name of Tylenol. Other brand name products include Feverall, Panadol, and Tempra (Table 1); generics are available as well. A few cough, cold, and allergy products specifically marketed for children also contain acetaminophen (Table 2), as do many adult over-the-counter cough, cold, and allergy products. The following name brand products for adults contain acetaminophen: Clear Cough Night Time; Comtrex Maximum Strength Liqui-Gel and Night Liquid; Decongestant Cough Formula A; Genite; Vicks Dayquil Multi-Symptom Cold/Flu Relief, 44M Soothing Cough, Cold, and Flu Relief, NyQuil Multi-Symptom Cold/Flu Relief; Night Time Cold Medicine, Flu, Cold, and Cough Medicine; Robitussin Night Relief; TheraFlu Maximum Strength Flu, Cold, and Cough Medicine NightTime, Flu, Cold, and Cough, and Flu, Cold; Tylenol Flu NightTime Maximum Strength Hot Medication, Cough with Decongestant Multi-Symptom, and Cold Multi-Symptom. Table 3 lists preparations marketed for adults that are available in liquid or powder form and so could easily be administered to infants, toddlers, and children.
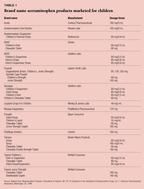


Since there are multiple preparations of acetaminophen, which vary in strength, and also multiple cough-and-cold preparations containing acetaminophen, errors in dosing may easily occur. Parents may not be aware that different formulations have different amounts of active ingredient, or they may be confused about how to calculate dosage by weight. They may not realize that that the cough-and-cold remedies they are using also contain acetaminophen. Or they may simply believe that more is better, especially with an OTC drug they think must be safe because it's so freely available.
Part of the problem has to do with the labeling on OTC preparations. Their language may exceed the reading comprehension of as many as 50% of parents. Labels for many preparations require eighth grade reading ability, while 19.6% of American adults have reading skills below the fifth grade level and 33% have skills between grades 6 and 10.13 In a study performed at the University of Rochester assessing 3,908 mother-initiated OTC medication doses, six pediatricians concluded that the mothers were often mistaken in what they believed to be the appropriate dose.14,15 In a recent study, 100 caregivers went through a mock dosing scenario in which they were asked to determine the appropriate dose and frequency of acetaminophen to give a child for fever. Caregivers were provided with product dosing information and their child's weight. Only 40% were able to determine the correct dose of acetaminophen for their children. Sixty-seven percent were able to measure the dose they planned to give correctly. Overall, only 30% of the caregivers both determined the proper dose and measured it accurately.16
As clinicians, it is essential that we teach parents about proper dosing of acetaminophen (see "Tips for clinicians about acetaminophen").
Tips for clinicians about acetaminophen
1. Encourage weight-based dosing (1015 mg/kg dose) not to exceed 5 doses/24 hours.
2. Be certain parents are familiar with differences in concentrations of various preparations. Encourage use of a single liquid preparation for all infants and children in the household.
3. Check cough/cold preparations to determine if acetaminophen is an ingredient.
4. Be careful with acetaminophen administration when given with drugs that stimulate P450 enzymes (phenobarbital, diphenylhydantoin, rifampin).
5. Avoid overzealous treatment of fever.
These points need to be addressed:
- Dosages are based on weight (10 to 15 mg acetaminophen/kg) and should not exceed five doses in a 24-hour period. Ask parents which acetaminophen preparation they are using and make dosage recommendations accordingly. Explain that formulations such as drops, liquid suspension, chewable tablets, and adult tablets differ substantially in the amount of acetaminophen they contain, and that if parents switch from one to another, they have to recalculate the dose.
- A child should receive a dosage form that is appropriate for his age. Infant drops are highly concentrated, so that a sufficient dose can be given in a small volume of liquid. They should be used only for infants less than 18 months of age. McNeil Consumer Healthcare has recently added the word "concentrated" to the label on Tylenol infant drops to signal caregivers that they contain more acetaminophen per unit of measure than does the liquid suspension form. The drops come with a dropper, which is the only measuring instrument that should be used. The suspension formulation comes with a small cup.
Suggest using the same liquid preparation for all infants and children in a household. If only the 160 mg/5 mL preparation were used, the increase in volume required for a correct infant dose, compared with the volume of the 80 mg/0.8 mL formulation (infant drops), would be relatively trivial. For example, for a 10 kg child, the dose would be 1.2 mL for drops and 3.75 mL for the suspension. Most parents should be able to get their infants to take this volume.
- Many cough-and-cold preparations contain acetaminophen. Emphasize the need to read labels, and explain to parents that, if they give their child a cough-and-cold medicine that contains acetaminophen and also give her plain acetaminophen, they need to do the necessary arithmetic to be sure the cumulative dose of acetaminophen is within the recommended range.
- Other medications such as phenobarbital, rifampin, and phenytoin can potentiate the effects of acetaminophen. Consider reducing the amount of acetaminophen recommended for children taking these medications, and explain the situation to their parents.
- Fever need not always be treated. Infants and children tolerate low fever well. Even when a fever is high and the child uncomfortable, there is no advantage to giving more acetaminophen than the recommended dose.17 If fever persists despite therapeutic dosages of acetaminophen, sponge bathing in tepid water is an effective way to reduce it.
As clinicians, we should be cautious in our stated or implied recommendations regarding an appropriate response to fever. Fever is generally protective, and pharmacologic efforts to reduce it may be harmful, particularly if they result in giving excessive acetaminophen. Relieving fever phobia in a parent may be one of our most effective, and safe, actions.
The bottom line
Based upon the small risk of hepatotoxicity from acetaminophen with chronic overdosing, should practitioners alter their choice of antipyretics? The answer is an unqualified No. Current evidence still suggests that acetaminophen has an acceptable safety profile. Practitioners in some communities recommend the use of ibuprofen because it provides a longer duration of antipyresis. Although the safety of ibuprofen appears well established, the safety profile is based upon insensitive measures such as hospitalization for gastrointestinal bleeding and renal failure. Ibuprofen has only been available as an OTC preparation since mid-1995, and it has not been as widely used as acetaminophen. As a nonsteroidal anti-inflammatory agent, it has potential gastrointestinal toxicity.
Some physicians have adopted the practice of alternating acetaminophen with ibuprofen, presumably for enhanced antipyresis. Both drugs are potentially nephrotoxic; their mechanisms of renal injury are different and may be synergistic. Therefore, it is appropriate to exercise caution in the practice of alternating doses.18,19
Until further information is available, it would appear judicious to continue using acetaminophen alone for antipyresis, along with sponge bathing for high fevers. At the same time, we should educate and reassure patients about the purpose of fever, its benefits, and reasonable treatment.
Acetaminophen is an effective antipyretic and analgesic when administered as recommended. Although care must be taken to avoid dosing mistakes and overzealous usage, the pharmacologic alternatives, including aspirin and ibuprofen, have significant potential toxicities that are not necessarily dose-related. Therefore, acetaminophen remains the drug of choice for antipyresis in infants and children.
ACKNOWLEDGMENT
The author wishes to acknowledge the careful editorial review and excellent suggestions provided by James Bien, MD, and support from Andrea Smith and Catherine McGraw in compiling this article. The research for this article was supported in part by the US Public Health Service Grant No. MO1RR08084 from the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health.
REFRENCES
1. Lesko SM, Mitchell AA: An assessment of the safety of pediatric ibuprofen. JAMA 1995;273:929
2. Proudfoot AT, Wright N: Acute paracetamol poisoning. BMJ 1970;3:557
3. Smilkstein MJ, Knapp GL, Kulig KW, et al: Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. New Engl J Med 1988;319:1557
4. Whitcomb DC, Block GD: Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994;272:1845
5. Rivera-Penera T, Gugig R, Davis J, et al: Outcome of acetaminophen overdose in pediatric patients and factors contributing to liver toxicity. J Pediatr 1997;130:300
6. Heubi JE, Bien JP: Acetaminophen use in children: More is not better. J Pediatr 1997;130:175
7. Heubi JE, Barbacci MB, Zimmerman HJ: Therapeutic misadventures with acetaminophen: Hepatotoxicity after multiple doses in children. J Pediatr 1998;132:22
8. Mitchell JR, Jollow DJ, Potter WZ, et al: Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 1973; 187(1):211
9. Roberts DW, Bucci TJ, Benson W, et al: Immunohistochemical localization and quantification of the 3-(Cystein-S-yl)-acetaminophen adduct in acetaminophen hepatotoxicity. Am J Pathol 1991;138:359
10. Lauterberg BH, Vaishnav Y, Stillwell WG, et al: The effects of age and glutathione deflection on hepatic glutathione turnover in vivo determined by acetaminophen probe analysis. J Pharmacol Exp Ther 1980;312:54
11. Nahata MC, Powell DA, Durrell DE, et al: Acetaminophen accumulation in pediatric patients after repeated therapeutic doses. Eur J Clin Pharmacol 1984;27:57
12. Amundi I, Lahteenmaki T, Rundrgren M, et al: Zonation of acetaminophen metabolism and cytochrome P4502E1mediated toxicity studied in isolated periportal and perivenous hepatocytes. Biochem Pharmacol 1993; 45:1251
13. Holt GA, Hollon JD, Hughes SC, et al: OTC labels: Can consumers read and understand them? Amer Pharmacy 1990: NS30:51
14. Maiman LA, Becker MH, Katlic AW: How mothers treat children's physical symptoms. J Commun Health 1985;10:136
15. Ackerman SJ: Doing more good than harm with children's medications. FDA Consumer 1989;23:28
16. Simon HK, Weinkle DA: Over-the-counter medications: Do parents give what they intend to give? Arch Pediatr Adoles Med 1997;151:654
17. Steele RW, Young FSH, Bass J, et al: Oral antipyretic therapy. Evaluation of aspirin-acetaminophen combination. Am J Dis Child 1972;123:204
18. McIntire SC, Rubenstein RC, Gartner JC Jr, et al: Acute flank pain and reversible renal dysfunction associated with nonsteroidal anti-inflammatory drug use. Pediatrics 1993; 92:459
19. Halpern SM, Fitzpatrick R, Volans GN: Ibuprofen toxicity: A review of adverse reactions and overdose. Adverse Drug React Toxicol Rev 1993;12:107
THE AUTHOR is Professor of Pediatrics, University of Cincinnati College of Medicine, and Director, Clinical Research Center, Children's Hospital Medical Center, Cincinnati.
James Heubi. Acetaminophen: The other side of the story. Contemporary Pediatrics 1999;12:61.