Acute otitis media: Update 2015
In May 2004, the American Academy of Pediatrics (AAP) joined with the American Academy of Family Physicians to publish a clinical practice guideline on the diagnosis and management of acute otitis media (AOM). In 2013, the AAP revised the guideline with important changes presented in this review.
Table 1

Figure 1
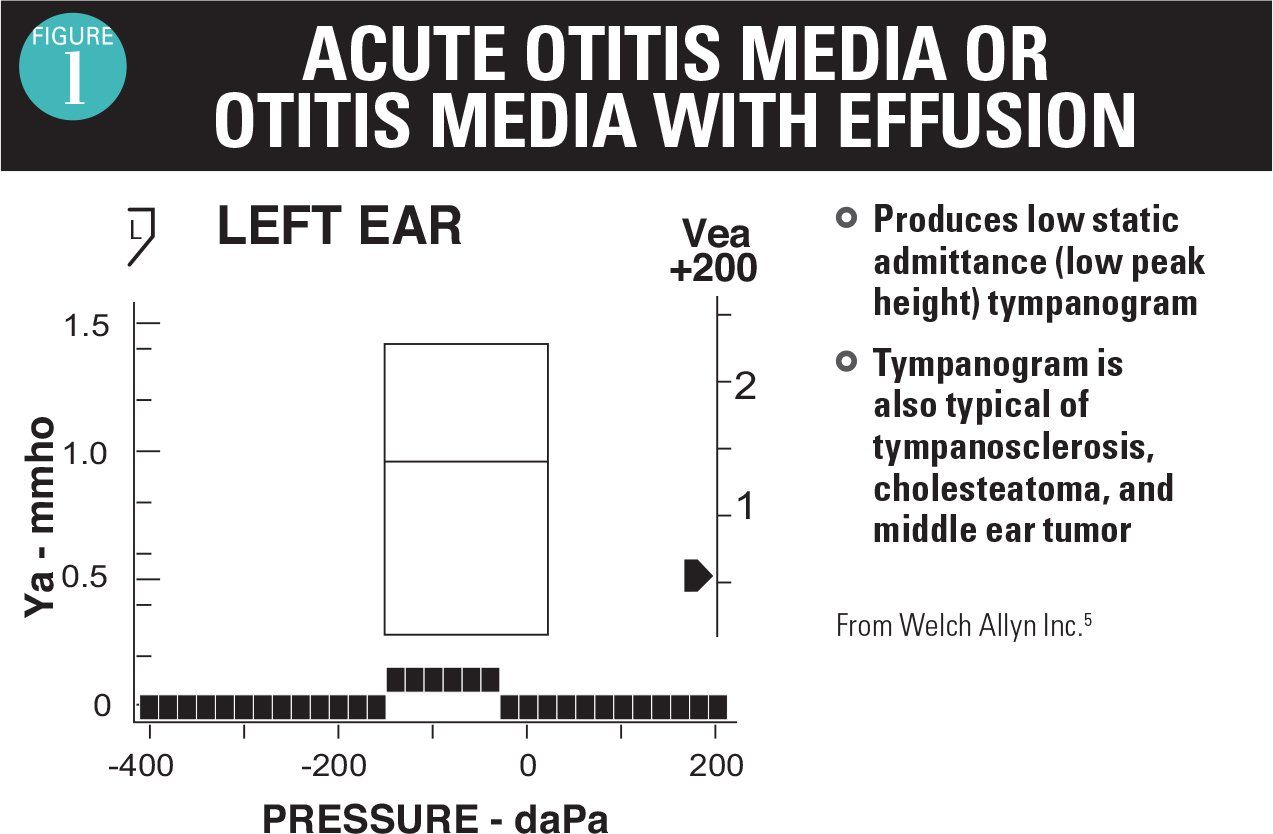
Figure 2
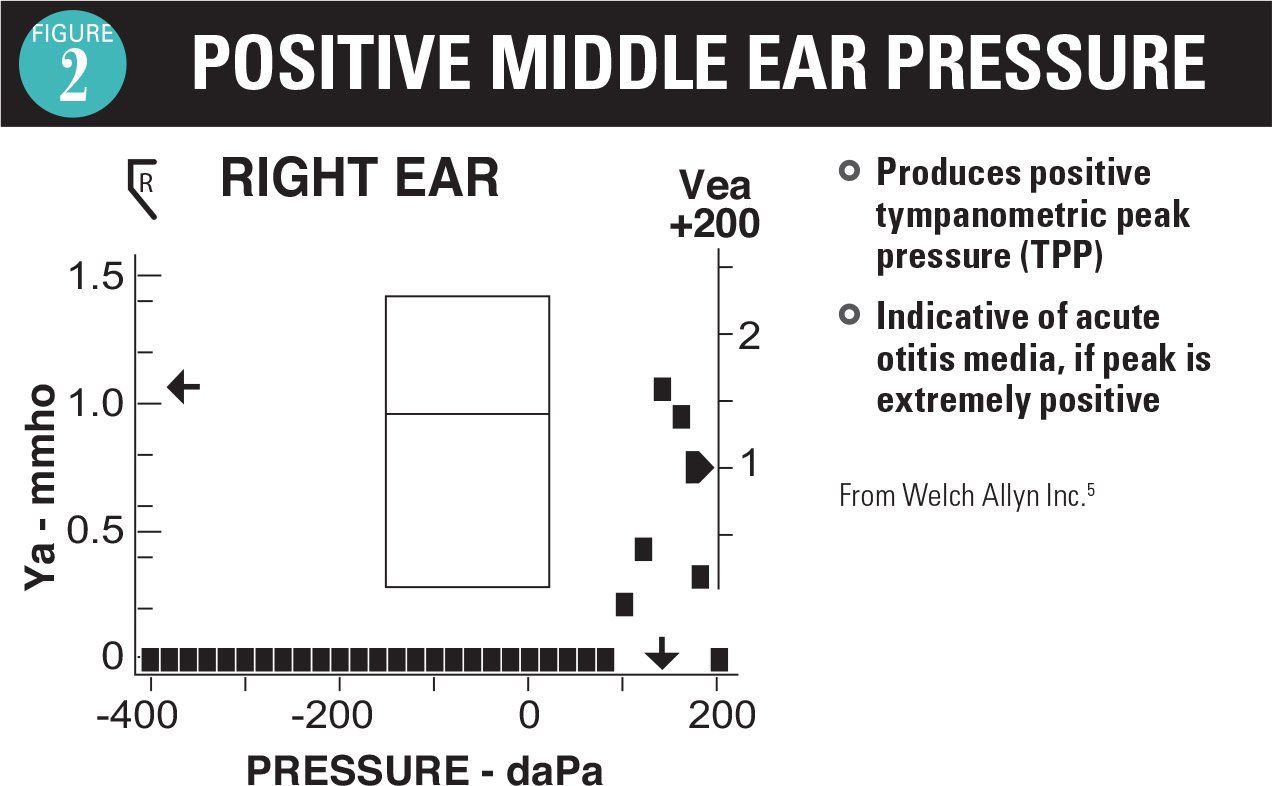
Figure 3
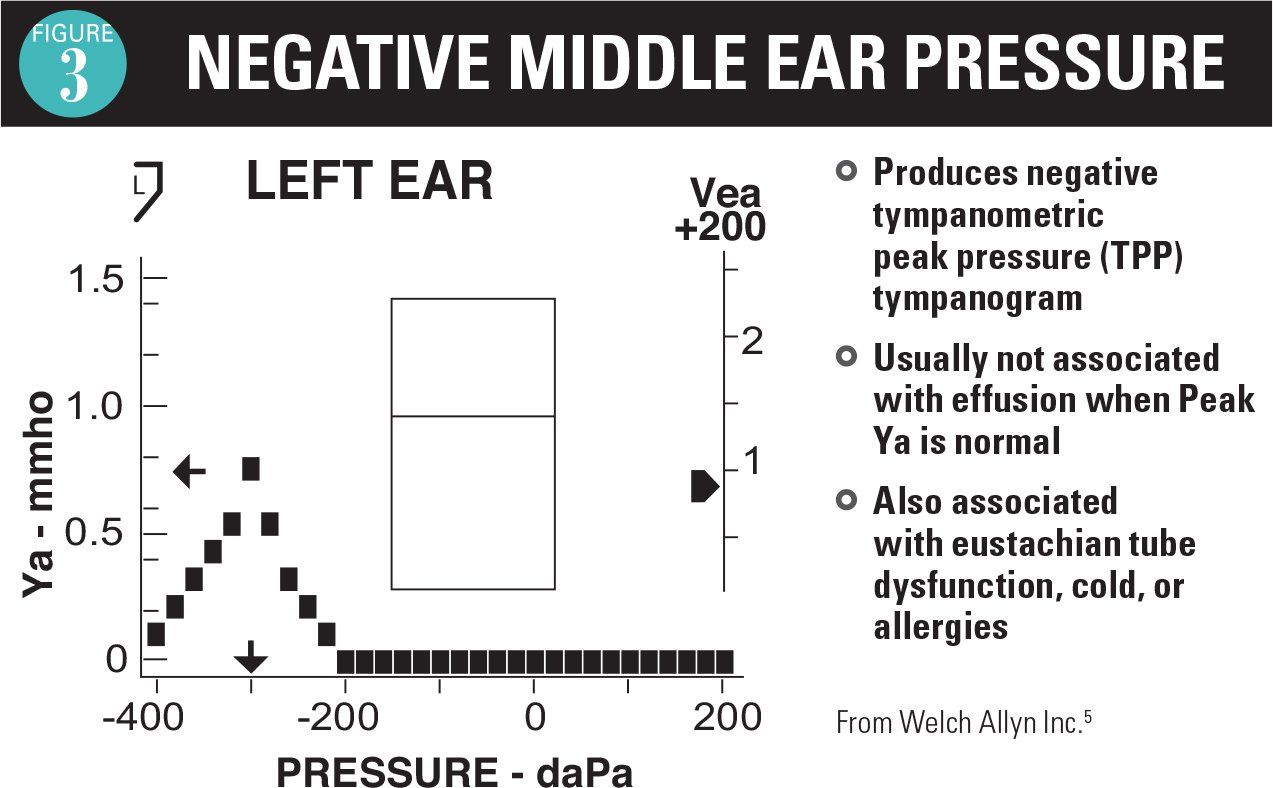
Table 2
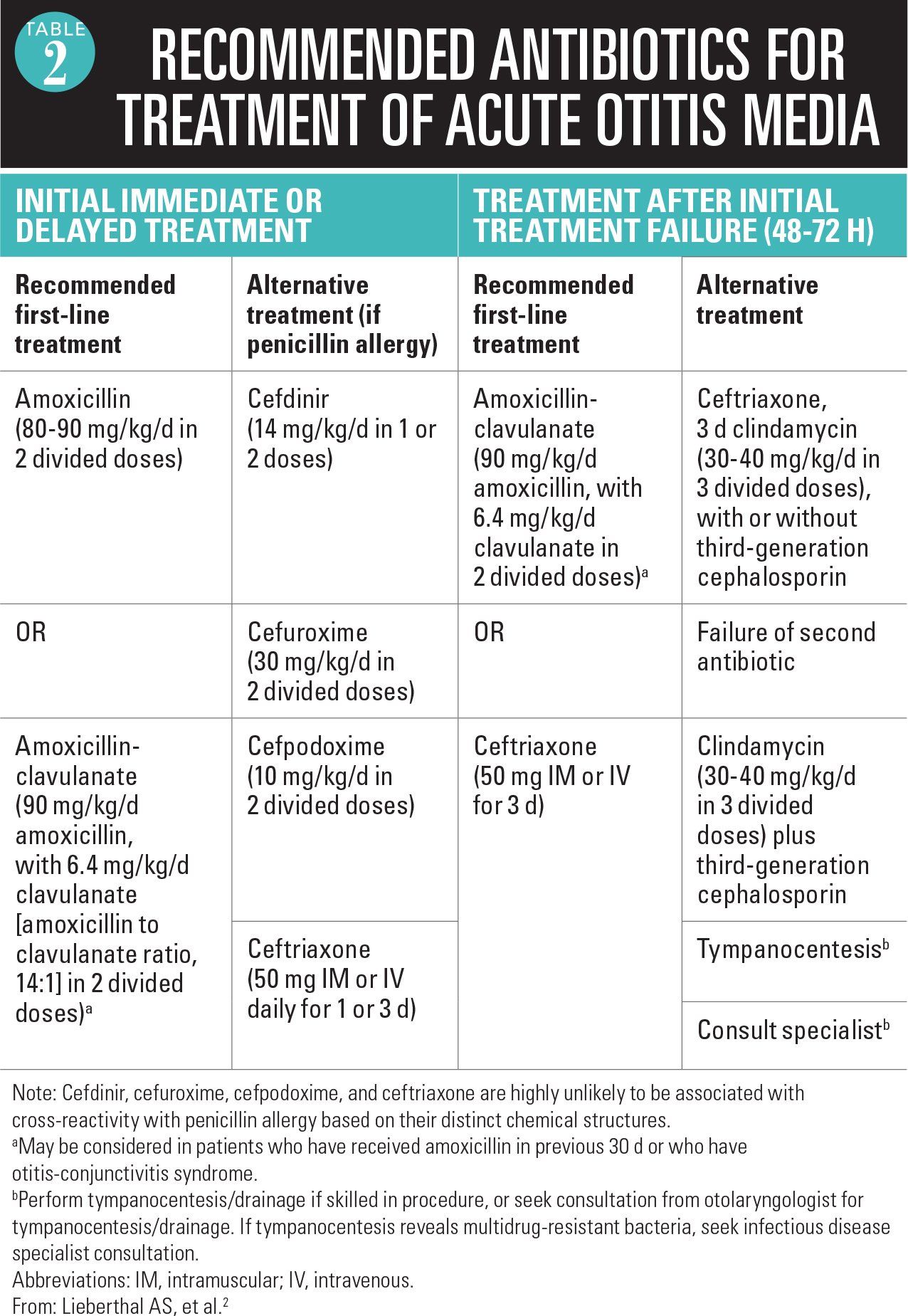
Table 3
In May 2004, the American Academy of Pediatrics (AAP) joined with the American Academy of Family Physicians to publish a clinical practice guideline on the diagnosis and management of acute otitis media (AOM).1 In 2013, the AAP revised the guideline with important changes presented in this review.2
The 2004 guideline was not without critics, and the same is true of the 2013 revision. The main areas of controversy surround diagnostic criteria for AOM, the notion of “uncertain diagnosis” and the watchful waiting option, and the selection of empiric antibiotic treatments recommended. Each of these issues will be discussed. We also discuss the otitis-prone child and the emergence of biofilms as a problem contributing to recurrent AOM and chronic otitis media with effusion (OME).
Diagnosis
The 2004 guideline criteria for diagnosis of AOM were insufficiently precise. Children with OME could fit the definition intended only to identify AOM. In the 2013 guideline, the diagnosis was refined and improved. Table 1 shows the key summary statements regarding diagnostic criteria from the 2013 guideline.2
The most important diagnostic feature for AOM noted in the new guideline is a bulging or full tympanic membrane (TM) associated with middle ear effusion, and the TM is opaque. Experts in otitis media diagnosis are in consensus supporting this change.3 The bulging occurs from pressure behind the TM caused by inflammation in the middle ear space. The AOM is not associated with a retracted TM, so a determination of retraction of the TM with middle ear effusion, regardless of TM opacity, is not AOM. A bulging compared with a retracted TM can be difficult to distinguish.
Because of the inflammation in the middle ear space during AOM, typically the TM becomes thickened and nontranslucent or completely opaque. A translucent TM is not seen with AOM. With a translucent or semitranslucent TM and middle ear fluid visualized behind the TM, the likely diagnosis is OME.3
Redness of the TM is not generally a valuable diagnostic sign of AOM. An exception would be the presence of a single red TM and the other TM not red, suggesting inflammation of the TM, consistent with the diagnosis of AOM if there is fluid visualized behind the TM. Most likely, such an examination represents an early AOM before inflammation has persisted to cause the TM to become more white or yellowish and opaque.
Acute otitis media is not associated with inflammation, so prior diagnostic definitions of OME stipulated absence of symptoms other than hearing loss. A child with OME may feel discomfort, however, and may feel popping noises that may cause ear tugging or even crying.4
Use of pneumatic otoscopy is very helpful to improve diagnostic accuracy, and its use is advocated in the 2013 guideline.2 Positive pressure on insufflation will result in movement backwards by a bulging TM, and negative pressure will result in movement forward by a retracted TM. Thus, properly used, pneumatic otoscopy can allow the clinician to have a better appreciation for the position of the TM, a key diagnostic feature.
An otoscope head that permits insertion of an insufflator is also needed. The challenge is to get a good seal with an otoscope speculum and to restrain the child long enough to perform the insufflation procedure. To achieve a seal, use of a speculum with a softer rubber sleeve midshaft of the speculum may be helpful.
As an adjunct to the otoscopic examination, 2 instruments are available as aids in the diagnosis of AOM and OME. A tympanometer identifies movement of the TM in response to positive and negative pressure. If no movement occurs with pressure, then the tympanogram readout is flat (Figure 1).5 This is the typical case for AOM and OME, so a flat tympanogram is abnormal but does not differentiate between AOM and OME. Infrequently, when the TM is becoming full and before it is bulging, a positive pressure tympanogram (Figure 2) is obtained. This occurs early in the AOM pathogenesis. In some cases, the TM will be retracted, giving a negative pressure tympanogram readout (Figure 3). A viral upper respiratory infection (URI), sometimes with concomitant middle ear fluid and sometimes OME, can result in a negative pressure tympanogram.
A disadvantage of tympanometry is that it requires a seal of the inserted speculum-like device in the external auditory canal. In children aged younger than 2 years, when AOM is most common, the child often moves and a seal cannot be obtained. Also, if a child is crying, a tympanogram reading cannot be obtained.
The sensitivity and specificity of tympanometry, using pneumatic otoscopy as a gold standard, has been recently assessed and shows that a normal tympanogram is very specific to rule out AOM and OME.6 An abnormal tympanogram should prompt a second visual look at the TM, because the test is not sensitive to detect AOM or OME.
Another instrument to assist in diagnosis of AOM and OME is the spectral gradient acoustic reflectometer (SGAR). This device relies on emittance of a sonar sound wave that bounces off the TM and back to the device to produce a reading. If the wave passes through a thickened TM, the wave is slowed giving a lower reading as occurs with AOM and OME. If the wave passes through fluid of any type, it slows the wave with thicker fluid (pus with AOM) impeding the wave more than thinner fluid (OME).
This instrument does not require a seal in the external auditory canal, and readings can be obtained in the crying child. The main limitation is the presence of wax in the external auditory canal. Cerumen that blocks the sonar wave emitted by the acoustic reflectometer is misinterpreted by the device, and false readings occur.
The sensitivity and specificity of SGAR, using pneumatic otoscopy as a gold standard, has been recently assessed and shows that a normal SGAR reading is very specific to rule out AOM and OME.6 Similar to the tympanogram, an abnormal reading should prompt a second visual look at the TM because the test is not sensitive to detect AOM or OME. The charges for tympanometry and SGAR are usually the same as reimbursed by most insurance companies. Most often, however, reimbursement is not available for both measurements on the same patient at the same visit.
Uncertain diagnosis and observation option
The notion that the clinical manifestations of AOM and OME are distinct from those of a viral URI s not accurate: AOM almost always occurs in the context of a viral URI, typically between the third and seventh day of a URI.7 Therefore, children with AOM and OME and a URI have rhinorrhea, nasal congestion, and elevated body temperature. When children have a viral URI, AOM, or OME, they are cranky, and they feed and sleep poorly. Clinicians (and parents) presume that a child pulling at the ears has an AOM. Even pulling the ear, however, is not a clear clinical manifestation of AOM or OME.4 When the TM becomes retracted, as often occurs with viral URIs, the change in position of the TM causes discomfort and ear tugging.
As a consequence of the vagueness of symptoms of AOM and their similarity to the symptoms of a viral URI or OME, as well as difficulties in visualizing the TM and performing pneumatic otoscopy, the recent AAP guideline maintains that sometimes there is an uncertain diagnosis of AOM.2 This is especially true in infants and young children, in whom symptoms are either mild or again overlap with the symptoms of a viral URI. Cerumen may certainly impede a proper visualization of the TM, which also may only show subtle changes. As well, apart from the factor of clinician inexperience with removing cerumen and performing pneumatic otoscopy, diagnostic equipment may be suboptimal and the child being evaluated may not always cooperate in the process.2
In those cases in which uncertainty occurs, if the child is not in pain and does not have a fever in excess of 102.2°F, then observation becomes an option.2 Utilizing observation as initial management in these instances requires the elements of easy follow-up and continuity of care. Absent either of those elements, the observation option is not advisable.
Empiric antibiotic treatment
The 2013 AAP guideline recommends high-dose amoxicillin (80-90 mg/kg/day in 2 divided doses) for 5 to 10 days as the treatment of first choice in most patients.2 Recommended optimal duration of therapy for children aged younger than 2 years and for those who have severe symptoms is a standard 10-day course; for children aged 2 to 5 years with mild or moderate AOM, a 7-day course appears adequate; and for those aged 6 years and older with mild or moderate AOM, a 5- to 7-day course is effective. The selection of high-dose amoxicillin has been made on the basis of long-term safety of the drug; a first intention-to-treat penicillin-resistant Streptococcus pneumoniae because that organism can cause the most morbidity; and the recognition that overdiagnosis of AOM is commonplace. This recommendation is unchanged from the 2004 guideline.1,2
An alternative recommended first-line treatment is amoxicillin-clavulanate (90 mg/kg/day amoxicillin and 6.4 mg/kg/day clavulanate in 2 divided doses). This alternative treatment is recommended in patients who have been given amoxicillin in the previous 30 days or in those who have otitis-conjunctivitis syndrome (Table 2).2
More: Child with fever after foreign travel
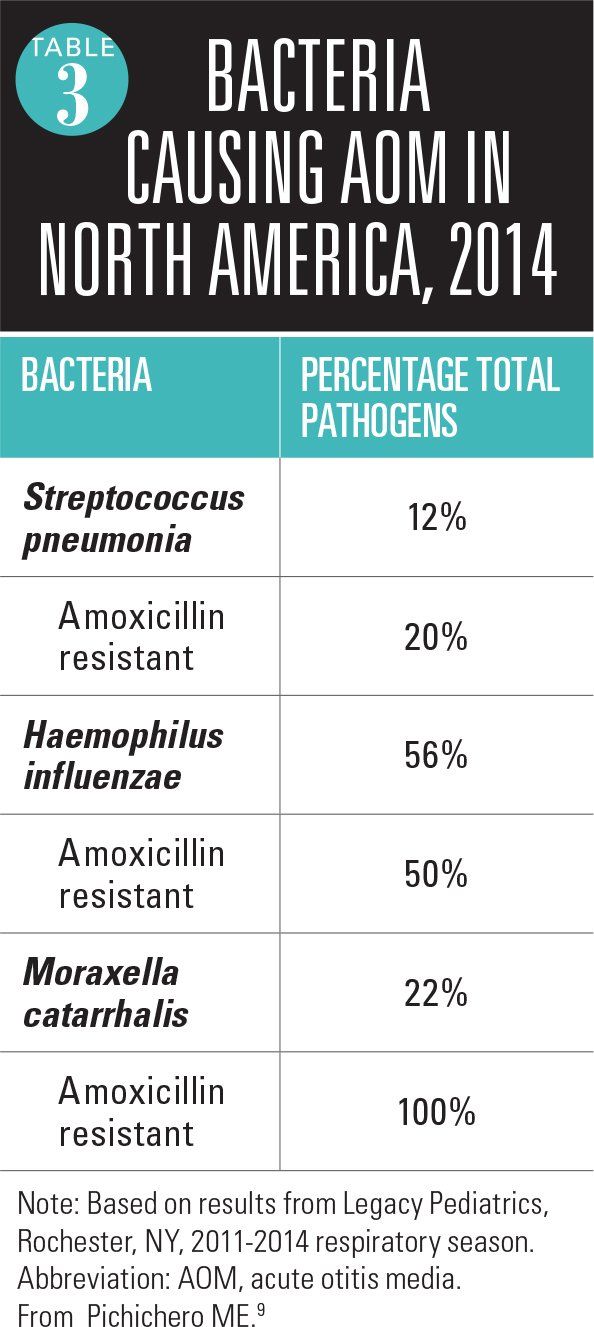
We have been tracking the etiology of AOM by performing tympanocentesis in our practice since 1996, before the introduction of 7-valent pneumococcal conjugate vaccine (PCV7). As a consequence of PCV7, and more recently PCV13 (substituted April 2010), the etiology of AOM has changed over time.8 Our data suggest that empiric amoxicillin treatment of AOM would be effective in eradication of about one-third of the current mix of otopathogens, because of the predominance of Haemophilus influenzae and Moraxella catarrhalis and their resistance to amoxicillin (Table 3).9 The diminished presence of S pneumoniae under vaccine-induced selection pressure may change over time, but in 2015 our data indicate that amoxicillin/clavulanate or a second- or third-generation cephalosporin with beta-lactamase stability should be the preferred treatment of AOM.There are no other data describing the otopathogen mix and antibiotic susceptibilities because our center currently is the only one in the United States routinely performing tympanocentesis with bacterial cultures of middle ear fluid. Two recent seminal studies that compared antibiotic to placebo and concluded that antibiotics were of significant benefit in hastening resolution of infection both used amoxicillin/clavulanate as the treatment choice.10,11 A third more recent study showed that antimicrobial treatment of AOM significantly hastened the disappearance of middle ear effusion in a placebo-controlled trial.12
The oral cephalosporins of choice for treatment of AOM as designated by the AAP guideline are cefdinir, cefuroxime axetil, and cefpodoxime proxetil.2 Among these choices, cefdinir has emerged as the most frequently used in the United States largely because of its taste advantage. Cefdinir can be dosed once or divided twice daily (14 mg/kg/day). The duration of treatment with cefdinir can be 5 days with twice-daily dosing or 10 days with once-daily dosing.
We recently published a head-to-head comparison of amoxicillin/clavulanate 80 mg/kg/day divided twice daily for 10 days versus cefdinir 14 mg/kg/day divided twice daily for 5 days. Amoxicillin/clavulanate demonstrated superior efficacy (86.5% vs 71%, respectively; P=.001).13 Outside the context of a clinical trial, however, the adherence characteristics favor cefdinir (better taste and fewer diarrhea episodes). The taste of cefuroxime and cefpodoxime can be masked with chocolate syrup, but the addition of flavorings at the pharmacy or at home should not be advised because these antibiotics have been shown to precipitate changes in pH and chemical reactions between the active drug and the flavoring ingredients.9
Ceftriaxone by injection (50 mg/kg/dose) is effective as a single injection against all penicillin-susceptible S pneumoniae and against beta-lactamase-producing H influenzae and M catarrhalis. Killing of penicillin-resistant S pneumoniae causing AOM has been proven to require 3 doses of ceftriaxone for 3 days.14 The sequential doses can be spaced every other day or even every third day if weekends or holidays dictate an alternative regimen.
Otitis-prone child
Some children experience repeated AOM episodes and reach a threshold where they are termed “otitis prone.” The definition of otitis prone has varied among investigators in the field. However, the most frequently used definition currently is 3 episodes of AOM within 6 months or 4 episodes within 1 year’s time. Children with recurrent AOM are generally treated with broader-spectrum antibiotics as additional cases of infection occur. We have recently shown that repeated antibiotic treatment does not change the mix of otopathogens causing AOM, but it significantly increases the proportion of strains that display amoxicillin resistance.15
In recent years, we have been studying the immune response to AOM to explain susceptibility to an increased frequency of AOM in otitis-prone children. We have discovered that the child who experiences frequent AOM has an immature immune system, resembling a neonate.16-20 After an AOM, the immune system of the otitis-prone child fails to generate an immune memory response. Therefore, the child remains susceptible to another AOM following antibiotic treatment, even by the same otopathogen residing in the nasopharynx that caused a preceding AOM.
Biofilms
When children have recurrent AOM or develop chronic OME (>3 months’ duration), there is now ample evidence to suggest that the otopathogens form biofilms.21,22 Bacteria in biofilms cover themselves with a shield of a plastic-like material that prevents the penetration of antibiotics and antibodies. Most clinicians are familiar with biofilms as bacteria that grow on implanted catheters. When bacteria are in biofilms, they do not elicit much of an immune response and therefore their presence is not apparent clinically.
We have recently shown that biofilms involving H influenzae require low oxygen conditions such as occur during chronic OME or recurrent AOM, but they do not form at normal oxygen concentrations.23 This new observation provides a new explanation as to why tympanocentesis and tympanostomy tubes provide therapeutic benefit.
Conclusion
The diagnosis of AOM should not be made unless the TM is full/bulging. Using pneumatic otoscopy, tympanometry, and/or SGAR should improve diagnostic accuracy. When the diagnosis of AOM is uncertain, the current AAP guideline proposes observation as an option and amoxicillin as empiric first-line treatment. The evidence does not support amoxicillin as the best treatment when the results of cultures of middle ear fluid taken in recent years are considered. Amoxicillin/clavulanate or a recommended cephalosporin should provide better efficacy. The biologic and immunologic basis of the otitis-prone condition points to new areas for research and alternative treatments.
REFERENCES
1. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451-1465.
2. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964-e999.
3. Shaikh N, Hoberman A, Kaleida PH, et al. Otoscopic signs of otitis media. Pediatr Infect Dis J. 2011;30(10):822-826.
4. Rothman R, Owens T, Simel DL. Does this child have acute otitis media? JAMA. 2003;290(12):1633-1640.
5. Welch Allyn Inc. Welch Allyn MicroTymp 2 Portable Tympanometric Instrument. User manual. Available at: http://intl.welchallyn.com/documents/EENT/Tympanometric%20Systems/MicroTymp%202/servicemanual_20070320_microtympII.pdf. Accessed February 6, 2015.
6. Puhakka T, Pulkkinen J, Silvennoinen H, Heikkinen T. Comparison of spectral gradient acoustic reflectometry and tympanometry for detection of middle-ear effusion in children. Pediatr Infect Dis J. 2014;33(8):e183-e186.
7. Revai K, Dobbs LA, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119(6):e1408-e1412.
8. Xu Q, Casey JR, Chang A, Pichichero ME. When co-colonizing the nasopharynx Haemophilus influenzae predominates over Streptococcus pneumoniae except serotype 19A strains to cause acute otitis media. Pediatr Infect Dis J. 2012;31(6):638-640.
9. Pichichero ME. Otitis media. Pediatr Clin North Am. 2013;60(2):391-407.
10. Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364(2):105-115.
11. Tähtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364(2):116-126.
12. Tapiainen T, Kujala T, Renko M, et al. Effect of antimicrobial treatment of acute otitis media on the daily disappearance of middle ear effusion: a placebo-controlled trial. JAMA Pediatr. 2014;168(7):635-641.
13. Casey JR, Block SL, Hedrick J, Almudevar A, Pichichero ME. Comparison of amoxicillin/clavulanic acid high dose with cefdinir in the treatment of acute otitis media. Drugs. 2012;72(15):1991-1997.
14. Gahanno P, Nguyen L, Barry B, et al. Eradication by ceftriaxone of Streptococcus pneumoniae isolates with increased resistance to penicillin in cases of acute otitis media. Antimicrob Agents Chemother. 1999;43(1):16-20.
15. Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32(8):805-809.
16. Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29(5):1023-1028.
17. Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30(8):645-650.
18. Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204(4):645-653.
19. Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205(8):1225-1229.
20. Sharma SK, Pichichero ME. Cellular immune response in young children accounts for recurrent acute otitis media. Curr Allergy Asthma Rep. 2013;13(5):495-500.
21. Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111(12):2083-2094.
22. Torretta S, Marchisio P, Drago L, et al. Nasopharyngeal biofilm-producing otopathogens in children with nonsevere recurrent acute otitis media. Otolaryngol Head Neck Surg. 2012;146(6);991-996.
23. Osgood R, Salamone F, Diaz A, et al. Effect of pH and oxygen on biofilm formation in acute otitis media associated NTHi clinical isolates. Laryngoscope. 2015; in press.
Dr Casey is a partner and co-director of research, Legacy Pediatrics, Rochester, New York, and clinical associate professor of pediatrics, University of Rochester. She has nothing to disclose in regard to affiliations with or financial interests in any organizations that may have an interest in any part of this article. Dr Pichichero, also at Legacy Pediatrics, Rochester, is director, Center for Infectious Diseases and Immunology, Rochester General Hospital Research Institute, and research professor, Health Sciences and Technology, Rochester Institute of Technology, New York. He discloses that he is a limited partner in a business entity that provided a loan to Innovia Medical LLC to make technical improvements in a spectral gradient acoustic reflectometer (SGAR) device.