Afrezza inhaled insulin meets primary endpoint in phase 3 pediatric diabetes trial
The primary endpoint was non-inferiority in HbA1c levels after 26 weeks.
Afrezza inhaled insulin meets primary endpoint in phase 3 pediatric diabetes trial | Image Credit: © Africa Studio - © Africa Studio - stock.adobe.com.

Positive phase 3 data for inhaled insulin Afrezza Inhalation Powder (MannKind Corporation) in children and adolescents aged 4 to 17 years has been announced by MannKind Corporation.1
According to the company, data from the phase 3 INHALE-1 study (NCT04974528) evaluating efficacy and safety of Afrezza met the primary endpoint of establishing the non-inferiority of Afrezza to multiple daily injections (MDI). With the positive data reported, MannKind intends to submit a request for a supplemental new drug application meeting with the FDA in the first half of 2025.1,2
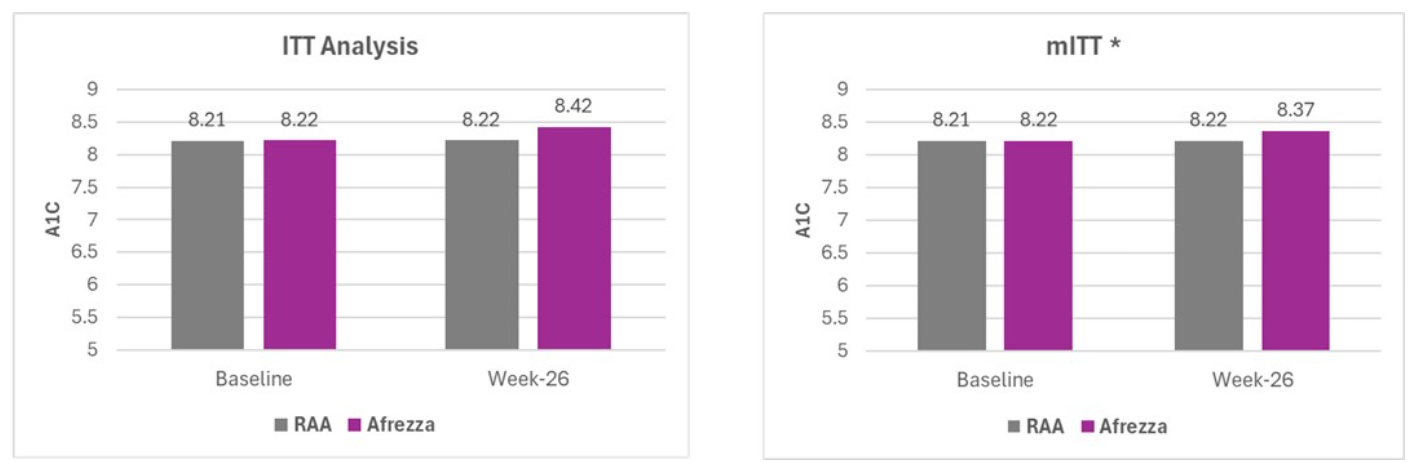
INHALE-1 is a 26-week, open-label clinical trial that features 230 individuals, who were randomized to Afrezza or MDI of rapid acting insulin analog in combination with basal insulin treatments in pediatric subjects with type 1 or type 2 diabetes mellitus. The primary endpoint was non-inferiority in HbA1c levels after 26 weeks.1,2
Between-group difference in mean HbA1c change over 26 weeks among the full intent-to-treat population (ITT) exceeded the pre-specified non-inferiority margin of 0.435%, "largely driven by the variability of a single patient who did not adhere to the study protocol," stated the company in a press release.
"A modified ITT (mITT) analysis, which excluded this subject, did not exceed the predetermined threshold of 0.4% (0.370%), thereby establishing the non-inferiority of Afrezza to MDI," said MannKind.
* mITT analysis excludes one outlier from the primary ITT endpoint who failed to adhere to the study protocol
Image courtesy of MannKind Corporation
Click to zoom
"The overall efficacy and safety outcomes seen in the first 26 weeks are encouraging," said Kevin Kaiserman, MD, senior vice president, Therapeutic Area head, Endocrine Diseases, MannKind Corporation, in a statement.
“It was exciting to partner with MannKind and help lead this study to potentially expand the use of inhaled insulin, which is currently used successfully by many adults with diabetes, to a population that hasn’t had a treatment option other than injectable insulin in the history of their care,” added Roy W. Beck, MD, PhD, founder of the Jaeb Center for Health Research who provided oversight for INHALE-1.
"The six-month results are clinically meaningful and show Afrezza as a potential future treatment option for a growing pediatric population living with type 1 and type 2 diabetes," said Beck.
References:
1. MannKind Announces Six-Month Results From Phase 3 INHALE-1 Pediatric Diabetes Trial Utilizing Inhaled Insulin (Afrezza). MannKind Corporation. Press release. December 16, 2024. Accessed December 19, 2024. https://investors.mannkindcorp.com/news-releases/news-release-details/mannkind-announces-six-month-results-phase-3-inhale-1-pediatric
2. Inhale. Clinical Trials. University of Florida Health. Updated December 19, 2024. Accessed December 19, 2024. https://ufhealth.org/clinical-trials/inhale