Boning up on common pediatric fractures
Appropriate evaluation and treatment of fractures of the distal radius, elbow, clavicle, and tibia hinge on the clinician?s recognition of mechanisms and patterns of injury and physical and radiographic findings.
Cover article
Boning up on common pediatric fractures
By M. Patrice Eiff, MD, and Robert L. Hatch, MD, MPH
Appropriate evaluation and treatment of fractures of the distal radius, elbow, clavicle, and tibia hinge on the clinician's recognition of mechanisms and patterns of injury and physical and radiographic findings.
Musculoskeletal injuries are frequently encountered in pediatric practice, and fractures account for a surprisingly large percentage of these injuries. Nearly 20% of children who present with an injury have a fracture and, it is estimated, 42% of boys and 27% of girls sustain a fracture during childhood.1,2 Common fracture locations include the distal radius, elbow, clavicle, and tibial shaft. Knowledge of normal bone growth and common injury patterns can be helpful to physicians who evaluate children with injuries. In this article, we review these general principles and discuss the assessment and management of selected fractures commonly encountered by pediatricians.
The growing bone: Anatomy and injury patterns
The major anatomic regions of growing bone include the epiphysis, physis, metaphysis, and diaphysis (Figure 1). The epiphysis is a secondary ossification center located at the end of long bones; it is separated from the rest of the bone by the cartilaginous physis. The age at which ossification centers become visible on a radiograph and the subsequent rates of physeal closure vary widely depending on the bone. The relative lack of ossification of many epiphyses in young children and the radiolucency of growth plates can make fracture identification difficult. Although comparison views of the uninjured side need not be obtained in every case, they can help you detect fractures in children when the mechanism of injury and clinical examination suggest a fracture.
PLEASE NOTE: If you are using Internet Explorer version 6 or higher, after clicking to enlarge each graphic in the article, click the picture icon that appears in the lower right-hand corner of the graphic.
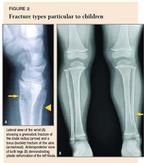
Injury patterns in growing bone. Immature bone has the ability to bow rather than break in response to force. This leads to fracture types not seen in adults. A compressive force in a child will produce a torus fracture, also called buckle fracture, instead of the impacted fractures that occur in adults (ulna in Figure 2A). A force applied to the side of a long bone may disrupt one cortex while merely bending the other, producing a greenstick fracture (radius in Figure 2A). In very young children, neither cortex may break, producing a bowing of the bone referred to as plastic deformation (Figure 2B).
The attachment of the physis to the metaphysis is a point of decreased strength in the bone and becomes the site of injury after musculoskeletal trauma in children. Ligaments and tendons are usually stronger than growing bone; in response to the same amount of injuring force, a child is more likely to fracture a bone, and an adult is more likely to tear a ligament, muscle, or tendon. A child's periosteum is thicker, stronger, and more biologically active than that of an adult and often remains intact following a fracture. The periosteum provides some tissue continuity across the fracture site that stabilizes the fracture and promotes more rapid healing.
Physeal injuries. Injuries to the physis (growth plate) constitute approximately 20% of all skeletal injuries in children.1 Damage to the physis can disrupt future growth at that site. The extent of growth disturbance is difficult to predict, because disruption of the physis itself causes slowing, whereas injury near but not involving the physis may actually stimulate growth. The most common growth disturbance after a physeal injury is premature partial arrest of growth.
Several factors determine the prognosis for physeal injuries. These include severity of injury (displacement, degree of comminution, open fracture), the patient's age, the particular physis injured, and the Salter-Harris radiographic type.3 Severity of injury is the most important factor. The Salter-Harris classification scheme is designed to stratify injuries according to their relative risk of growth disturbance4 (Figure 3). Type II fractures are by far the most common (approximately 50% of all physeal fractures), followed, in descending order, by types I, III, IV, and V. As a whole, types I and II have a low risk of growth disturbance, and the relative risk increases from type II to V. However, it is important to consider all factors when assessing the risk of growth disturbance and to remember that growth disturbance can occur after any fracture in the region of a physis.
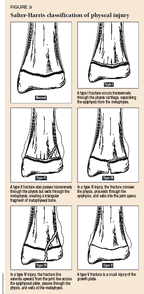
In our opinion, children with a type III, IV, or V fracture should be referred to an orthopedic surgeon for definitive care. Nondisplaced type I and II fractures can be managed effectively by a pediatrician because these fractures usually heal well with closed treatment. Even though the risk of growth arrest is minimal, both type I and II fractures should be followed radiographically to ensure that normal growth has resumed. Monthly radiographs for three months are adequate follow-up after most type I fractures; six months of surveillance is recommended for most type II fractures.
Educating the patient and the parent regarding the possibility of growth disturbance after a physeal injury is essential and should be initiated when the fracture is first diagnosed. They should be advised of the likelihood of growth disturbance (based on the Salter-Harris type and severity of injury), that growth disturbance may occur with any fracture of the physis (regardless of the treatment), and that consistent follow-up to monitor bone growth is very important.
Healing and remodeling of fractures. Pediatric fractures typically heal much more quickly than adult fractures. This can be advantageous: Children typically require shorter immobilization times. A disadvantage, however, is that malpositioned fragments become immovable much earlier than in adults. Whereas a clinician may have eight to 10 days in which to detect and correct unacceptable fragment position in an adult, you may have only three to five days in a young child and five to seven days in an older child to do this.
The normal process of bone remodeling in a child may correct malalignment, making near-anatomic reductions less important in children than in adults. Remodeling can be expected if the patient has two or more years of bone growth remaining. Rotational deformity remodels poorly, if at all, and should be corrected by reduction. Mild angular deformities often correct themselves, however, as the bone grows. The deformity is most likely to correct itself if the child is younger, the fracture is closer to the physis, and the angulation is in the same plane of motion as the nearest joint. Because the amount of remodeling is not predictable, displaced fractures should still be reduced to maximize the chances of achieving acceptable alignment. It is best to refer patients with displaced fractures to an orthopedic surgeon for reduction and definitive treatment.
Fractures in children may stimulate longitudinal growth of the bone, which may make the bone longer than it would have been had it not been injured. Some degree of fracture fragment overlap and shortening is, therefore, acceptable and even desirable in certain fractures to counterbalance the anticipated overgrowth. This is particularly true for fractures of the femoral or tibial shaft.
Children tolerate prolonged immobilization much better than adults. Disabling stiffness or loss of range of motion is distinctly unusual after pediatric fractures. After cast immobilization, physical therapy is rarely needed because children tend to resume their normal activity gradually without much supervision. Playing in a swimming pool may speed up return to full function, if desired. Even though fractures of growing bones generally heal with a large callus, this new bone is still fibrous and not yet restored to its original strength. Because of this, the child should avoid collisions or contact activities for two to four weeks, depending on activity level and age, after discontinuing immobilization.
Distal radial fractures
The distal radius is perhaps the most common fracture site in children and adolescents. The incidence of fractures of the distal forearm has increased 40% over the last 30 years, with most of the increase occurring in fractures associated with recreational activities.5 Fractures of the metaphysis account for 62% of distal radial fractures, and the distal radial physis is the most commonly injured physis in the body.1,6 The discussion in this section, therefore, focuses on these two groups of fractures.
Metaphyseal fractures. The peak incidence of distal radial fractures coincides with the peak growth velocity for children, because of the relative porosity of the bone during this time. The usual mechanism of injury is a fall on the extended wrist. A simple fall may result in a nondisplaced fracture, whereas a fall in conjunction with forward momentum (as when, for example, the child is riding a bicycle or in-line skating) is more likely to produce a displaced fracture. Dorsal displacement of the distal fracture fragment may be disguised by soft-tissue swelling. Check sensation of the fingers carefully for any sign of median or ulnar nerve injury. Nerve injury is more likely if the fracture is significantly angulated or if there is significant swelling at the fracture site. Make sure you do a complete exam of the elbow and wrist because distal radial fractures may be associated with supracondylar or scaphoid fractures.
Anteroposterior (AP) and lateral views of the wrist are usually adequate to determine the type of fracture and amount of displacement. It is important to distinguish torus (buckle) fractures from greenstick and complete fractures involving both cortices. Torus fractures are usually nondisplaced because of the strong intact periosteum and can be managed by a pediatrician. These fractures may be very subtle and seen more easily on the lateral view (Figure 4). Greenstick fractures and complete fractures usually show compression of the dorsal cortex and apex volar angulation (Figures 2A and 5A). These fractures are at risk of late displacement or reangulation (Figure 5B) and should be referred to an orthopedist. Distal radial fractures are rarely isolated, so check the radiographs carefully for an associated ulnar styloid or distal ulna metaphyseal fracture.
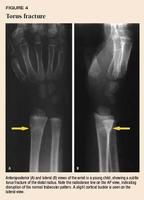

With torus fractures, reduction is unnecessary unless angulation exceeds 15°. If the injury is more than 48 hours old, a short arm cast can be applied safely at the first visit. Use a short arm volar splint for the first few days if the injury is less than 48 hours old to allow for any swelling. These fractures heal well with virtually no complications after immobilization for two to four weeks in a short arm cast. A removable volar splint that provides comfort and protects from re-injury is an acceptable alternative to a short arm cast.7 Repeat radiographs to document healing are unnecessary unless the child is not clinically healed after four weeks of immobilization. To reduce the risk of re-injury, it is advisable for the patient to wear a volar splint during vigorous activity for an additional two weeks.
Nondisplaced greenstick fractures can be treated with a short arm cast. Angulation of more than 15° should be corrected by closed reduction followed by immobilization in a long arm cast.8 Obtain follow-up radiographs three to seven days after injury and then every one to two weeks thereafter to be sure position is maintained. If the fracture has become more angulated, refer the patient to an orthopedist for treatment. Greenstick fractures should be healed after four weeks of cast immobilization. Protection with a volar splint during vigorous activity is a good idea for an additional two weeks after the cast is removed.
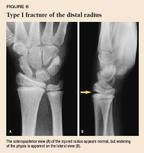
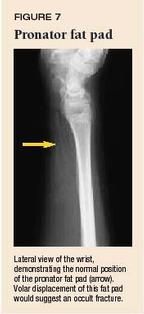
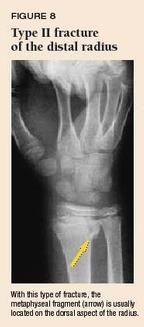
Physeal fractures. A nondisplaced Salter-Harris type I fracture of the distal radius physis can be difficult to detect radiographically (Figure 6). The presence of the pronator fat pad along the volar aspect of the distal radius on the lateral view (Figure 7) is a reliable sign of an occult fracture, although its absence does not rule out a fracture.9 If there is marked localized tenderness over the physis, treat the child as having a physeal injury even if radiographs are normal. In a type II physeal fracture, the metaphyseal fragment is most often located on the dorsal aspect of the radius (Figure 8). If the epiphysis is going to displace, it usually displaces dorsally.
Nondisplaced fractures can be treated with a short arm cast for three to six weeks depending on the age of the childthe older the child, the longer the immobilization. A volar splint may be worn for one or two more weeks for both comfort and protection, and to allow range of motion and strengthening exercises as the child returns to full activity. Follow-up radiographs at three to six months are essential to document normal growth.
The child with physeal tenderness but normal radiographs should be immobilized in a short arm cast for two weeks and then reevaluated. If repeat radiographs show callus formation, immobilization should be continued for an additional two weeks. If the radiographs are normal at the two-week visit, a physeal injury is unlikely and the cast can be discontinued. Displaced physeal fractures should be reduced as soon as possible by an experienced clinician. The longer the delay in reduction, the greater the force that must be applied to obtain an adequate reduction, which puts the physis at greater risk of injury and subsequent growth arrest.
Elbow fractures
Fractures to the elbow represent approximately 10% of all fractures in children. Diagnosis and management of these injuries are complex, and most fractures should be managed by an orthopedist. The challenge for the pediatrician is to promptly recognize those elbow injuries that require referral. The large amount of nonossified cartilage around the elbow makes detection of fractures difficult. Elbow fractures in children have the potential for neurovascular injury, which further complicates management.
More than half of all pediatric elbow fractures are supracondylar. These, combined with lateral condylar fractures, make up more than 80% of elbow fractures in children.10 In the newborn, nearly the entire elbow joint is cartilaginous (nonossified) and therefore not visible on radiographs. The sequence of ossification of the various growth centers can be memorized using the mnemonic "Come Read My Tale Of Love," which stands for the capitellum, radial head, medial epicondyle, trochlea, olecranon, and lateral epicondyle (see the mnemonic guide below). Ossification of these growth centers occurs at approximately 1, 3, 5, 7, 9, and 11 years of age, respectively. Knowing this sequence can help you distinguish a fracture from a normal finding in the elbow. If in doubt, obtain comparable views of the uninjured side for comparison.
"Come Read My Tale Of Love"
(an ossification mnemonic for the elbow)
Important aspects of the clinical examination of a child with an elbow injury include careful observation of his movement of the injured arm, thorough neurovascular examination, and examination of the wrist and shoulder to detect associated injuries. The child's ability to flex and extend the fingers and the wrist and to oppose the thumb and little finger are good indicators of intact motor function. The vascular examination should include palpation of the brachial and radial pulses and an assessment of capillary refill in the fingers.
Before getting radiographs, immobilize the elbow with medial and lateral splints to prevent potential injury from sharp fracture fragments during positioning for the radiographs. The best position for splinting is the elbow flexed 20° to 30°, which places the least tension on neurovascular structures. Initial AP and lateral views can be obtained in this position to diagnose a displaced fracture. Definitive radiographs in the proper positions can be obtained after this initial screen once the patient is more comfortable and has received appropriate acute treatment.
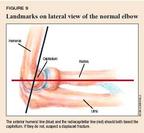
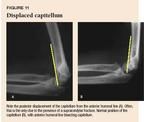
Fractures and displacement of nonossified portions of the elbow are difficult to detect radiographically. Comparison views and additional oblique views may help you distinguish a fracture from a growth center.11,12 Knowledge of some basic landmarks on the lateral view will help you in your review of elbow radiographs. The anterior humeral line, drawn parallel to the anterior edge of the humerus, should course through the middle third of the capitellum (Figure 9). The radiocapitellar line, drawn through the axis of the radial shaft, points directly to the capitellum in all views. Disruption of these normal anatomic relationships indicates a displaced fracture. The fracture line may not be apparent in a nondisplaced supracondylar fracture, and a positive fat pad sign may be the only clue to an occult fracture13 (Figure 10).

Supracondylar fractures. The weakest part of the elbow joint is the supracondylar area where the humerus flattens and flares. The most common type of supracondylar fracture is the extension-type, in which the hyperextended position drives the olecranon into the supracondylar portion of the humerus, resulting in a fracture.
Children with a supracondylar fracture have marked pain and swelling of the elbow. If the fracture is significantly displaced, there will be obvious deformity of the joint. Kinking or pressure on the artery, if present, usually causes vascular compromise. Therefore, a pulse can usually be re-established by simply reducing the fracture. The brachial artery is at risk of injury from the displaced fracture fragments, but vascular injury requiring surgical intervention is rare. Compartment syndrome of the forearm can complicate this fracture, so be alert to signs of increasing pressure, such as unrelenting pain, severe swelling, and paresthesia. Test sensation and motor function of the hand to detect any nerve injury. Nerve injury after a supracondylar fracture is usually caused by stretching or contusion of the nerve. This usually resolves spontaneously within several weeks.
Supracondylar fractures in children are classified as type I (nondisplaced or minimally displaced), type II (displaced distal fragment with an intact posterior cortex), or type III (displaced with no contact between fragments). The anterior humeral line is abnormal in most even minimally displaced supracondylar fractures (Figure 11A). Displaced supracondylar fractures are well visualized on the lateral view (Figure 12).
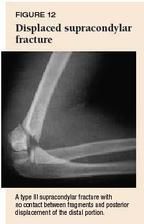
Most supracondylar fractures in children are displaced and need surgical intervention. Nondisplaced (type I) fractures can be managed by immobilizing the arm in a long arm cast with the forearm in neutral rotation and the elbow at 90° for four weeks. If the fracture is acute, the cast should generally be bivalved to avoid creation of an iatrogenic compartment syndrome as swelling progresses. Obtain radiographs three to seven days after injury to document maintenance of normal alignment and then at four weeks to document callus formation. At this point, discontinue the cast and have the child start active range of motion. Suboptimal healing is more likely in cases of incomplete reduction, severe displacement, and extensive soft tissue damage. Supracondylar fractures can be complicated by malunion, most commonly a varus angulation of the elbow with loss of full extension ("gunstock" deformity). This rarely creates a functional deficit but may be of cosmetic concern. In severe cases, surgical correction with osteotomy is indicated.
Lateral condylar fractures are the second most common elbow fracture and the most common physeal elbow injury. With a fall on an outstretched hand combined with a varus force, the lateral condyle may be avulsed. The most characteristic finding is localized swelling over the lateral distal humerus. In the most common pattern, the fracture line begins in the distal humeral metaphysis and extends just medial to the capitellar physis into the joint (Figure 13). Neurovascular injury is a rare complication of these fractures.
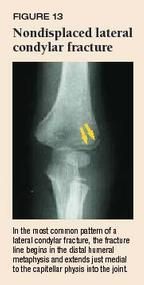
Because this fracture is intraarticular, open reduction is required to restore normal alignment in displaced fractures. Although nondisplaced or minimally displaced fractures can be treated by a pediatrician, referral may be preferable in most cases. These fractures can be treated acutely with a posterior splint with the forearm in neutral position (no pronation or supination, with the wrist in a position of function, e.g., as if holding a glass in the hand) and the elbow at 90°. Continue the posterior splint and obtain a repeat radiograph weekly to check for any late displacement. If the fracture remains nondisplaced after two weeks, apply a long arm cast for another four to six weeks or until there is evidence of radiographic union. Growth arrest and nonunion are the most common complications and can result in a cubitus varus or valgus deformity. Overstretching in the presence of a cubitus valgus deformity can cause a late ulnar nerve palsy.
Clavicular fractures
Most fractures of the clavicle (80%) occur in the middle third of the bone; another 15% involve the distal third, and 5% involve the proximal third. Common mechanisms of injury include a fall on an outstretched hand, fall onto the shoulder, or direct trauma to the clavicle. The patient with a fracture of the middle third of the clavicle usually complains of pain with any shoulder movement and holds the arm against the chest to protect against motion. The diagnosis of fracture is generally straightforward because of obvious downward and inward deformity of the shoulder. On examination, there is point tenderness over the fracture site. Crepitus or palpable motion of the fracture fragments is not uncommon given the subcutaneous position of the clavicle.
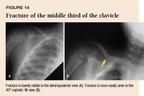
An AP view is usually the only radiograph required to accurately diagnose a midshaft fracture. If the diagnosis is in question, a 45° cephalic tilt view can uncover the fracture (Figure 14). In a displaced fracture, the strong pull of the sternocleidomastoid muscle results in upward displacement of the medial portion of the clavicle, while the lateral fragment is pulled downward by the weight of the arm. In a younger child, plastic bowing of the clavicle may occur. This injury should be treated just like other fractures to prevent an overt fracture.
In our experience, a sling works as well as a figure-of-eight bandage in treating clavicular fractures, both displaced and nondisplaced; use whichever is more comfortable (usually a sling). Consider the fracture clinically healed when the fracture site is painless and the patient can move the arm fully without discomfort. The sling or figure-of-eight bandage can be discontinued when it is no longer providing comfort. Orthopedic follow-up is not recommended. Most children are back to full activity by four weeks after injury. Protection from contact or collision sports is advisable for an additional two or three weeks following adequate clinical and radiographic healing. Complications are rare.14 Young children tend to produce an exuberant callus at the fracture site, producing an easily visible bulge. You can spare parents some worry by warning them in advance that this might happen.
The clavicle may be fractured during the trauma of childbirth. These fractures usually involve the midshaft. Neonatal clavicular fractures are easily detected if you have a heightened level of suspicion for this lesion after a difficult delivery, especially when complicated by shoulder dystocia. The infant with a clavicular fracture has decreased use of the arm on the injured side and reacts with pain to movement of the arm. Manipulation of the clavicle usually produces crepitus or palpable motion at the fracture site. Ultrasonography can confirm the diagnosis if you want to avoid the radiation exposure of plain radiography.15 Little treatment is needed for newborn clavicular fractures because they almost always heal well within two weeks. Avoiding pressure on the clavicle and minimizing movement of the affected arm during feeding, dressing, and handling ensure greater comfort. Pinning the sleeve of the affected side to the chest with the elbow at 90° is another treatment option. Once symptoms resolve, the infant will begin to use the arm normally.
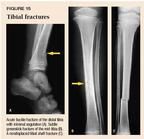
Tibial fractures
Although isolated fractures of the tibia are common in children, concurrent fractures of the tibia and fibula are even more common. Because the management of both bone fractures is much more complicated and usually requires referral, you must carefully scrutinize the entire length of the fibula for a coexisting fracture. The fibular fracture may be of the plastic deformity type, which can be easily missed (Figure 2).
Pediatric tibial fractures may be of the torus (buckle) type, the greenstick type, or complete (Figure 15). Compared to fractures of both the tibia and fibula, isolated fractures of the tibia are usually caused by relatively mild forces. Falls and twisting injuries of the foot are common mechanisms of injury. The combination of low force, intact periosteum, and support from the intact fibula prevent significant displacement in most cases. Displaced fractures, concurrent fractures of the tibia and fibula, and fractures with greater than 15° of varus angulation should be referred. Other children with nondisplaced fractures are treated with a long leg cast for six to eight weeks. Repeat radiographs weekly to check fracture position, and refer the patient at the first sign of increasing angulation. Mild varus deformity is not uncommon and should remodel if it is less than 15°.

Toddler's fractures occur most commonly in children younger than 2 years old who are learning to walk. Frequently, there is no definite history of a traumatic event, and the child is brought to the office because of reluctance to bear weight on the leg. Examine the hip, thigh, and knee first to rule out other causes of limping. [Editor's note: For more on this topic, see "A practical approach to the child who limps," in the February 2002 issue.] A thorough physical examination may be limited by the child's cooperation, but maximal tenderness can usually be elicited over the fracture site.
If you suspect a toddler's fracture, obtain AP and lateral views of the entire tibia and fibula. The typical findings are a nondisplaced spiral fracture of the tibia and no fibular fracture (Figure 16). It is not uncommon for initial radiographs to be normal and the diagnosis of this fracture made several days after the injury when follow-up radiographs show a lucent line or periosteal reaction.
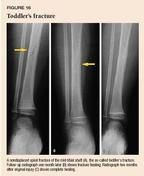
Distinguishing a toddler's fracture from a fracture caused by child abuse may be somewhat difficult because there is often no witnessed trauma and the spiral fracture suggests a twisting force. Examine the child carefully for signs of suspicious soft tissue trauma, such as bruises on the buttocks and the back of the legs, head, and neck. Transverse fractures of the mid-shaft and bone fractures of the tibia and fibula are more often caused by abuse and should arouse greater suspicion.
A toddler's fracture should be immobilized in a long leg cast for three to four weeks. Weight bearing is allowed as tolerated. If the fracture is discovered as a late finding (two or more weeks after injury), immobilization may not be necessary unless the child is quite symptomatic. The fracture usually heals completely within six to eight weeks.
Fractures of abuse
A majority of fractures in children younger than 1 year are caused by physical abuse, and a significant percentage of the fractures in children younger than 3 years are the result of abuse.16 Although all children are potentially at risk of maltreatment and abuse, first-born children, premature infants, stepchildren, and children with learning or physical disabilities are at greater risk.
When evaluating a child with a musculoskeletal injury, take a thorough history to assess the possibility that the injury was not accidental. Delineate and document the specifics surrounding the episode of trauma carefully. Observe the parents' telling of the history, their reaction to the event, their interaction with their child, and their cooperativeness with the health-care team carefully for signs of evasiveness, vagueness, or changing details of the circumstances of the injury.
It is not unusual for young children to fall, but it is unusual for them to sustain a significant injury from the fall alone. It is rare for an infant to sustain a fracture from a fall from a sofa or changing table.17 You must decide whether the reported history of the trauma is consistent with the pattern, severity, and extent of the injury. Knowledge of the developmental abilities of children and of the usual mechanisms of various injuries and fractures can be essential in making the appropriate diagnosis. If you are uncertain of the usual mechanism of a given injury, consult a fracture management text.18,19
There is no predominant fracture pattern in child abuse. Spiral fractures of the long bones have for years been thought to be highly suggestive of child abuse. In reality, diaphyseal fractures caused by abuse can be transverse, oblique, or spiral; the pattern is determined by the force applied. Fractures caused by physical abuse are most often found in the humerus, femur, and tibia. Other sites of fractures of abuse are (in descending order of frequency) the radius, skull, spine, ribs, ulna, and fibula.
Femoral fractures in children younger than 1 year are highly suspicious for child abuse (Table 1). Because scapular fractures result only after significant force, a scapular fracture in a child without a clear history of violent trauma should raise suspicion of abuse. Other fracture patterns associated with child abuse include epiphyseal and metaphyseal fractures of the long bones and corner or "chip" fractures of the metaphyses. Radiographic evidence of unexplained fractures in different stages of healing is perhaps the strongest finding suggestive of child abuse.
TABLE 1
Which fractures should make you suspicious of child abuse?
Unexplained fractures in different stages of healing as shown on radiography
Femoral fracture in a child younger than 1 year
Scapular fracture in a child without a clear history of violent trauma
Epiphyseal and metaphyseal fractures of the long bones
Corner or "chip"fractures of the metaphyses
A skeletal survey should be considered in the evaluation of a young child who may have been physically abused. The American Academy of Pediatrics has published recommendations for diagnostic imaging of child abuse.20 The skeletal survey is the mainstay of diagnosis in infants. A bone scan is a useful complementary study and is often used as the principal imaging tool beyond 1 year of age.21 Bone scans are, however, limited in their ability to reveal epiphyseal, metaphyseal, and skull fractures, and they are not useful in dating fractures.
Informed fracture management
Fractures are a common childhood injury. Being knowledgeable about injury patterns, typical mechanisms of injury, and physical and radiographic findings helps ensure adequate evaluation and treatment. The pitfalls and complications associated with a fracture vary with its location, type, and displacement. Understanding the pertinent considerations, as outlined in this discussion, allows you to sort fractures into those likely to have an excellent outcome with conservative treatment and those that require referral (Table 2). Using the guidelines discussed in this article, pediatricians can confidently manage many common fractures.
TABLE 2
Fractures that warrant referral to an orthopedist
REFERENCES
1. Wilkins KE: The incidence of fractures in children, in Rockwood CA, Wilkins KE, Beaty JH (eds): Fractures in Children, ed 4, vol 3, pp 317, Philadelphia, Lippincott-Raven, 1996
2. Landin LA: Fracture patterns in children. Acta Orthop Scand (Supp 202) 1983;54:1
3. Peterson HA: Physeal and apophyseal injuries, in Rockwood CA, Wilkins KE, Beaty JH (eds): Fractures in Children, ed 4, vol 3, pp 120121. Philadelphia, Lippincott-Raven, 1996
4. Salter RB, Harris WR: Injuries involving the epiphyseal plate. J Bone Joint Surg 1963;45A:587
5. Khosla S, Melton LJ, Dekutoski MB, et al: Incidence of childhood distal forearm fractures over 30 years. A population based study. JAMA 2003;290:1479
6. Peterson CA, Peterson HA: Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma 1972;12:275
7. Solan MC, Rees R, Daly K: Current management of torus fractures of the distal radius. Injury 2002;33:503
8. Noonan KJ, Price CT: Forearm and distal radius fractures in children. J Am Acad Orthop Surg 1998;6:146
9. Musharafieh RS, Macari G: Salter-Harris I fractures of the distal radius misdiagnosed as a wrist sprain. J Emerg Med 2000;19:265
10. Townsend DJ, Bassett GS: Common elbow fractures in children. Am Fam Phys 1996;53:2031
11. Chacon D, Kissoon N, Brown T, et al: Use of comparison radiographs in the diagnosis of traumatic injuries of the elbow. Ann Emerg Med 1992;21:895
12. Kissoon N, Galpin R, Gayle M, et al: Evaluation of the role of comparison radiographs in the diagnosis of traumatic elbow injuries. J Pediatr Orthop 1995;15:449
13. Skaggs DL, Mirzayan R: The posterior fat pad sign in association with occult fracture of the elbow in children. J Bone Joint Surg (Am) 1999;81:1429
14. Calder JD, Solan M, Gidwani S, et al: Management of pediatric clavicle fracturesIs follow-up necessary? An audit of 346 cases. Ann R Coll Surg Engl 2002; 84:331
15. Kayser R, Mahlfeld K, Heyde C, et al: Ultrasonographic imaging of fractures of the clavicle in newborn infants. J Bone Joint Surg [Br] 2003;85-B;115
16. Cramer KE, Green NE: Child Abuse, in Green NE, Swiontkowski MF (eds): Skeletal Trauma in Children, ed 2, vol 3. Philadelphia, WB Saunders, 1998, p 578
17. Helfer RE, Slovis TL, Black M: Injuries resulting when small children fall out of bed. Pediatrics 1977;60:533
18. Eiff MP, Hatch RL, Calmbach WL: Fracture Management for Primary Care, ed 2. Philadelphia, WB Saunders, 2003
19. McRae R: Practical Fracture Treatment, ed 4. Philadelphia, Churchill Livingstone, 2002
20. Sane SM, Kleinman PK, Cohen RA, et al: American Academy of Pediatrics. Section on Radiology. Diagnostic imaging of child abuse. Pediatrics 2000;105:1345
21. Nimkin K, Kleinman PK: Imaging of child abuse. Radiol Clin North Am 2001;39:843
DR. EIFF is associate professor, department of family medicine, Oregon Health & Science University, Portland, Ore.
DR. HATCH is associate professor, department of community health and family medicine, University of Florida College of Medicine, Gainesville, Fla.
The authors have nothing to disclose in regard to affiliations with, or financial interests in, any organization that may have an interest in any part of this article.
M. Patrice Eiff, Robert Hatch. Boning up on common pediatric fractures.
Contemporary Pediatrics
November 2003;20:30.