Endocrine nondisease: Pituitary and other imposters
These guidelines will help you identify childhood syndromes that masquerade as pituitary, parathyroid, and thyroid disorders and one that mimics Marfan syndrome. Second of two parts.
Endocrine nondisease:
Pituitary and other imposters
By Michael S. Kappy, MD, PhD
These guidelines will help you identify childhood syndromes that masquerade as pituitary, parathyroid, and thyroid disorders and one that mimics Marfan syndrome. Second of two parts.
A"nondisease" exists when a constellation of physical and laboratory findings suggests a specific disease or syndrome that is not present. Many children referred for evaluation for suspected endocrine disorders turn out to have a nondisease instead. The first part of this article ("Is it an endocrine disorder or a 'nondisease'?" October 2000) explained how to differentiate conditions that mimic adrenal, carbohydrate, and gonadal disease from true disease. This article describes syndromes that resemble pituitary, parathyroid, and thyroid disorders, as well as one that imitates Marfan syndrome.
Pituitary nondisease
Syndromes that may masquerade as pituitary diseases include:
Familial short stature/familial tall stature. Abnormalities in a child's stature and growth velocity often lead to a suspicion of hypopituitarism (growth hormone or thyroid-stimulating hormone- thyroid-hormone deficiency) in the short child or growth hormone excess in the tall child. The majority of these children, however, fall within a normal distribution of growth velocities based on the average of their parents' heights. Normal prepubertal growth velocities vary widely for age, from approximately 4.5 to 7.5 cm/year between 4 and 10 years of age, depending on familial stature. During these years, children from relatively short families tend to grow at significantly lower rates than children from tall families.
Differences in the final heights of children are caused in part by the differences in their growth velocities for several years before the pubertal growth spurt. The same differences in growth velocities between children from short and tall families usually exist during puberty. Consequently, the final heights of these children differ by many centimeters.
If the physician is reasonably sure that the child shows no sign of chronic illness or central nervous system abnormality and is growing within the normal range for his or her skeletal maturation, additional evaluation is not usually indicated. If, however, the doctor suspects abnormal growth, particularly in a short child, a basic laboratory evaluationincluding a complete blood count, urinalysis, electrolytes, renal profile, and thyroid function studies (free T4 and thyroid stimulating hormone [TSH]) may help establish a cause. If all of these values are normal, then abnormal height (short or tall) in the face of normal growth velocity for mid-parental stature suggests only familial short or tall stature and not pituitary disease.
Obesity in infancy (cultural/familial). We are often asked to evaluate young children (6 months to 5 years of age) with an excessive growth rate for the presence of a growth hormone-secreting tumor of the pituitary gland. Figure 1 shows the growth pattern from birth to 3.4 years of age of just such a child along with a photo of the child at 2 years of age. His growth rate was approximately 8.5 cm/year from 1.5 to 3.4 years of age, which was elevated for chronologic age (upper limit of normal = 7.5 cm/year). His stature and growth velocities also were abnormal for his family, since the mean of his parents' heights was only 168 cm (about 66 inches). The average height in the United States is 172 cm (about 68 inches).
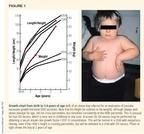
A significant family history of obesity and type 2 (noninsulin-dependent) diabetes mellitus existed. Both parents were about 50% overweight. His mother had gestational diabetes, and the child was born large for gestational age at 9 lb (4.1 kg).
By 2 months of age, the boy showed generalized obesity, and his weight age (the age for which his weight was the 50th percentile) exceeded his height age. He was more than twice his ideal weight-for-height at the time of referral. His morning serum cortisol concentration was normal, as were his thyroid function and blood glucose, but his fasting insulin concentration was elevated at 39 µU/mL.
Children such as this patient do not, as a rule, have a growth hormone-secreting tumor but are more appropriately considered postnatal equivalents of infants of diabetic mothers. Their intake is generally excessive, reflecting cultural/familial patterns that result in obesity and often type 2 diabetes. The resultant elevated fasting insulin-to-glucose ratio seen in many of these children is a marker of their already hyperinsulinemic state and insulin resistance and confirms the diagnosis of "nonpituitary disease."1-3 If additional laboratory evaluation is needed, a normal serum insulin-like growth factor-I (IGF-I) concentration for age will differentiate children with cultural/familial obesity from those with abnormal growth hormone secretion. A cranial MRI is almost never necessary.
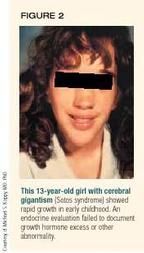
Cerebral gigantism (Sotos syndrome) is a nonendocrine cause of abnormally tall stature with rapid growth in early childhood.4,5 It is often confused with states of growth hormone excess because of the characteristic early, rapid growth and slightly "acromegaloid" facial appearance. Children with Sotos syndrome appear normal on endocrinologic evaluation with no abnormality of the growth hormone/IGF-I axis or other endocrine function. They have variable degrees of developmental delay and a characteristic facies (Figure 2) with a relatively large head, which usually distinguish them from children with pituitary lesions that hypersecrete growth hormone. The condition is thought to be inherited as an autosomal dominant trait.6 It usually occurs sporadically, however. Occasionally, a normal serum IGF-I concentration is necessary to rule out growth hormone excess in children with cerebral gigantism.
Gynecomastia in adolescent males. Unilateral or bilateral breast enlargement in adolescent males is a relatively common finding, occurring in around 30% of normal boys.7 The differential (pathologic) diagnosis includes Klinefelter syndrome, prolactinoma, and drugs that may increase the circulating concentration of prolactin (such as antidopaminergic antidepressants and marijuana). Trauma, infections, and tumors should also be included in the differential diagnosis, but are uncommon. Rarely, a boy will report galactorrhea or visual difficulties, which should prompt a more rigorous evaluation. Generally, however, serum prolactin concentrations are normal, even in boys who have findings compatible with Klinefelter syndrome, such as a eunuchoidal body build and small, firm testes.
Serum prolactin may be moderately elevated (30 to 70 ng/mL) in the presence of gynecomastia if a boy gives a history of marijuana or antidepressant use, but pituitary disease as a cause of the gynecomastia is rare. Prolactin-secreting micro- and macroadenomas of the pituitary are most uncommon in this age group. They usually reveal themselves through circulating concentrations of prolactin that are greater than 80 ng/mL. Serum prolactin concentration can be determined if suspicion of pathology exists, but cranial MRI is rarely necessary and is never appropriate as a first step.
Parathyroid nondisease
Children suspected of having parathyroid disease usually come to the attention of the physician because of an abnormal laboratory test result. The physical examination is usually normal.
Benign hyperphosphatasemia, which is marked by isolated elevation of alkaline phosphatase, is seen most often in infants, but has also been documented in adults.8,9 It is usually transient, but may persist for years, especially in familial forms.10,11 The differential diagnosis of elevated alkaline phosphatase includes a variety of hepatic and skeletal metabolic disorders (such as rickets) and hyperparathyroidism, either primary or caused by renal disease.
Benign hyperphosphatasemia is characterized by the following8:
- Isolated elevation of alkaline phosphatase, often greater than 10,000 IU/L. The abnormality is usually discovered during a routine laboratory workup for other conditions, such as poor growth in infancy. The majority of the elevated alkaline phosphatase is the skeletal isozyme.
- There is no clinical evidence of liver or bone disease.
- The elevated alkaline phosphatase usually returns to normal within 12 to 20 weeks, although longer times to resolution have been reported.10,11
The evaluation of such children should initially be limited to laboratory studies and should not include bone or liver scans. If concentrations of serum calcium, phosphorus, and a simultaneous serum parathyroid hormone are normal, then the diagnosis of parathyroid nondisease can be made (Table 1). In most cases, if the child has a normal physical examination and growth rate and the clinical impression is of a "well child," the alkaline phosphatase does not have to be monitored unless new concerns about the patient's status arise.
TABLE 1
Lab results in true hyperparathyroidism vs. parathyroid nondiseases
Benign familial hypercalcemia. Occasionally, the physician will discover a mildly elevated serum calcium concentration (11.0 to 12.0 mg/dL or 5.5 to 6.0 mEq/L) in a child during a routine laboratory evaluation. The serum phosphorus concentration is usually normal or slightly low, and the serum parathyroid hormone concentration is in the "normal" range, but high for the serum calcium. The hallmark of this condition is low urine calcium excretion.12 When a random urine specimen is obtained, the calcium-to-creatinine ratio (both expressed in mg/dL) is low for age,13,14 which distinguishes benign familial hypercalcemia from the elevated urine calcium excretion and very low serum phosphorus concentration commonly seen in hyperparathyroidism.
Benign familial hypercalcemia requires no treatment since it does not lead to nephrocalcinosis or other morbidity. It is inherited as an autosomal dominant trait and may be documented in one of the parents. The basis of the condition is a nonfunctioning mutation in the gene(s) for calcium sensors on the cell surface of the parathyroid glands and kidney.14 The genes for various forms of this condition have been localized to the long arms of chromosome 3 and 19.15,16 As a result of the abnormal receptor, the parathyroid glands and the kidneys do not respond normally to circulating serum calcium concentrations. Parathyroid hormone thus continues to be secreted at a serum calcium concentration that would ordinarily suppress it (that is, a new, higher set-point is present). Likewise, the kidneys do not respond to the elevated serum calcium by increasing its excretion, and hypocalciuria results. Because of the usual finding of low calcium excretion, this condition is also known as familial hypocalciuric hypercalcemia.
Thyroid nondisease
Nondisease syndromes that mimic thyroid disorders include congenital nonhypothyroidism, acquired nonhypothyroidism, and nonhyperthyroidism.
Congenital nonhypothyroidism. There are at least three examples of this condition, the first two of which result from normal thyroid hormone physiology. The third is caused by the relatively nonsensitive and nonspecific nature of the physical examination in newborns with true congenital hypothyroidism.
During delivery and immediately after the umbilical cord is cut, infants experience a profound surge in TSH secretion (Figure 3). This enables newborns to generate increased energy and heat to better adjust to the rapid change in ambient temperature from that of the uterus (37° C) to that of the delivery room (24° C). If an initial screening is performed in the first 24 hours of life, as is common, the baby's circulating TSH concentration may be elevated compared to normal screening values, which are generally reported as less than 20 mU/L by most state laboratories. Values of 20 to 50 mU/L in the first 24 hours of life may reflect nothing more than this neonatal TSH surge and not congenital hypothyroidism, particularly if the total thyroid hormone concentration (T4) is normal.
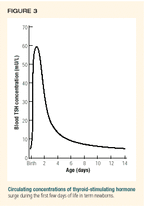
A study of 122,000 infants born in Colorado and Wyoming from 1998 to 1999 found no infants with congenital hypothyroidism whose TSH concentration was less than 45 mU/L on initial screening.17 Congenital hypothyroidism was most likely if the initial TSH was greater than 60 mU/L, with 45 to 60 mU/L being a gray zone. In states that have initiated mandatory second screening at 10 to 14 days of life, this artifact disappears, since the early TSH surge subsides by the third day of life. Any newborn suspected of having true primary congenital hypothyroidism should have a complete thyroid profile within the first two to three weeks of life.
The second example of congenital nonhypothyroidism results from a deficiency of thyroid-binding globulin (TBG), which is usually inherited autosomally as a clinically normal condition. Since thyroid hormone is transported in the blood by a series of proteins, predominantly TBG, decreases in TBG reduce the circulating concentration of total T4. Decreased TBG levels do not, however, reduce the active or free form of T4 (free T4), which regulates the secretion of TSH. In TBG deficiency, therefore, total T4 is low but free T4 is not, and TSH is not elevated as it is in true primary congenital hypothyroidism (Table 2). TBG deficiency is as common as true primary congenital hypothyroidism and requires no treatment. Table 2 also describes two examples of true hypothyroidism without elevations in circulating TSH concentrations.
TABLE 2
Congenital primary hypothyroidism compared with nonhypothyroidism and two other hypothyroid conditions
In some hospitals, free T4 measurement is unavailable, and the T3 resin percentage uptake (T3RU) is used as a measure of TBG's saturation with T4. In true hypothyroidism, the T3RU is low along with the total T4, whereas in TBG deficiency, it is high. The resultant calculated free thyroxine index (FTI), the total T4 multiplied by the T3RU, is normal, implying that the free T4, if measured, would be normal. Most hospital laboratories can now measure circulating free T4 concentrations, which, combined with measurement of TSH, are preferred over the combination of total T4, T3RU, and TSH for evaluating children with suspected thyroid disease.
The third example of congenital nonhypothyroidism affects newborns with one or more of the following findings on physical examination in whom congenital hypothyroidism is suspected: large anterior or posterior fontanelle, large tongue, jaundice, mottled skin, umbilical hernia, or hypotonia. Studies of newborns with documented congenital hypothyroidism have shown that only 25% to 30% have any of the above findings, indicating a 25% to 30% sensitivity for the physical examination.18,19 More important, the findings are hardly specific for congenital hypothyroidism because they are relatively common, and congenital hypothyroidism occurs in only about one of every 5,000 births in this country. Determining circulating thyroid hormone and TSH concentrations by means of newborn screening with appropriate laboratory confirmation of suspected abnormal results remains the only reliable way to diagnose true congenital hypothyroidism.
Acquired nonhypothyroidism. The same tall, obese children who are referred for evaluation for possible Cushing syndrome also are often referred for evaluation for aquired hypothyroidism, since a deficiency of thyroid hormone would predispose the child to obesity. As a rule, if the child is at an age when growth velocity can be used as a criterion, normal growth is evidence against long-standing hypothyroidism. In addition, these children tend to have a normal or slightly advanced skeletal maturation for age, whereas children with hypothyroidism have delayed skeletal maturation.
Symptoms generally associated with hypothyroidism include fatigue, sluggishness, cold intolerance, dry skin, and constipation, but these are not always seen in hypothyroid children and may be seen in children with "simple" obesity. The symptoms are, therefore, neither sensitive nor specific for hypothyroidism, and the tall, obese child with a normal to advanced skeletal maturation most often has acquired nonhypothyroidism as well as non-Cushing syndrome. When in doubt, a thyroid panel (free T4 and TSH or T4, T3RU, and TSH) should be obtained.
Nonhyperthyroidism. TBG excess can also result in thyroid nondisease. In this condition, circulating concentrations of total T4 are elevated, while free T4 is normal. Therefore, TSH is not suppressed as it is in true hyperthyroidism (Table 3), and the child is asymptomatic. TBG excess can be inherited autosomally, or it can be caused by the relative increase in circulating estrogen concentration that occurs during pregnancy and in women taking estrogen supplements alone or in combination with a progestational agent.
TABLE 3
True hyperthyroidism compared with nonhyperthyroid conditions
Another nonhyperthyroid condition suspected on the basis of abnormal laboratory results is the rare, autosomal dominant resistance to thyroid hormone. In this condition, the peripheral and central (hypothalamic/pituitary) T4 receptors show less affinity for T4 than normal. The resultant clinical picture is characterized by excessive secretion of T4 (high total and free T4) without suppression of TSH. This effect is caused by inadequate feedback inhibition centrally. The absence of hyperthyroid symptoms results from the relative resistance of peripheral receptors to T4. The lack of suppression of TSH and the absence of symptoms in T4 resistance and TBG excess should lead the physician to suspect "nonhyperthyroidism" in both conditions.
Non-Marfan syndrome
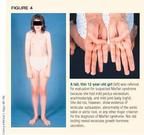
Another nondisease seen by the endocrinologist involves the tall, thin child with long fingers and toes and mild joint laxity. Mild pectus excavatum may be present, and the child may be able to bring his or her thumb completely across the palm (Figure 4). Marfan syndrome, an autosomal dominant condition that is inherited 75% of the time and that occurs sporadically 25% of the time, is often considered in patients like this, but the diagnosis is rare.
Mutations in the gene for fibrillin-1 (FBN-1) on chromosome 15 have been found in many, though not all, individuals with Marfan syndrome, 20,21 but also in individuals who do not have the syndrome. Thus, a sensitive or specific genetic marker for this condition does not yet exist, and the diagnosis must be made on clinical grounds. According to the most recent diagnostic criteria for Marfan syndrome, subluxation of the lens or evidence of abnormal cardiovascular anatomy (such as aortic valve disease or aortic root dilatation) and less specific skeletal or other system abnormalities must be present to make the diagnosis in the absence of a positive family/genetic history (Table 4).22 A positive family history of Marfan syndrome is suggestive, but not diagnostic. Disorders such as homocystinuria must still be ruled out.
TABLE 4
Diagnostic criteria for Marfan syndrome
Skeletal system
"Involvement" of the skeletal system requires at least two of the major criteria or one major criterion plus two minor criteria
Major criteria (at least 4 of the following manifestations)
Pectus carinatum
Pectus excavatum requiring surgery
Reduced upper-to-lower ratio or arm span-to-height ratio >1.05
Wrist and thumb signs
Scoliosis >20º or spondylolisthesis
Reduced extension at the elbows (<170º)
Medial displacement of the medial malleolus causing pes planus
Protrusio acetabulae of any degree (ascertained by radiographs)
Minor criteria
Pectus excavatum of moderate severity
Joint hypermobility
High arched palate with crowding of teeth
Facial appearance (dolichocephaly, malar hypoplasia, etc.)
Ocular system
"Involvement" of the ocular system requires the presence of at least two of the minor criteria
Major criterion
Ectopia lentis
Minor criteria
Abnormally flat cornea (determined by keratometry)
Increased axial length of globe (measured by ultrasound)
Hypoplastic iris or hypoplastic ciliary muscle causing decreased miosis
Cardiovascular system
"Involvement" of the cardiovascular system requires the presence of a major or minor criterion
Major criteria
Dilatation of the ascending aorta with or without aortic regurgitation and involving at least the sinuses of Valsalva
Dissection of the ascending aorta
Minor criteria
Mitral valve prolapse with or without mitral valve regurgitation
Dilatation of the main pulmonary artery in the absence of valvular or peripheral pulmonic stenosis or any other obvious cause below 40 yr of age
Calcification of the mitral annulus below 40 yr of age
Dilatation or dissection of the descending thoracic or abdominal aorta below 50 yr of age
Family/genetic history
One of the major criteria must be present for the family/genetic history to be contributory
Major criteria
A parent, child, or sibling of the patient who meets the diagnostic criteria independently
Presence of a mutation in FBN-1 known to cause Marfan syndrome
Presence of a haplotype around FBN-1, inherited by descent, known to be associated with unequivocally diagnosed Marfan syndrome in the family
Diagnosis of Marfan syndrome
For the index case:
If the family/genetic history is not contributory, major criteria in at least two different organ systems and involvement of a third system is required
If a mutation known to cause Marfan syndrome in others is detected, one major criterion in an organ system and involvement of a second system is required
For a relative of an index case:
Presence of a major criterion in the family history and one major criterion in an organ system and involvement of a second system is required
The majority of children referred for evaluation for Marfan syndrome do not have the condition. Considering the emotional and psychological toll of the syndrome and its impact on medical insurance, excluding the diagnosis in a timely fashion is just as important as identifying the syndrome is to beginning prompt, appropriate health supervision.23
Avoiding unnecessary testing and worry
The concept of "nondisease" faded from the literature after its initial report in 1965 and a subsequent report in 1974 regarding nonhypoglycemia.24,25 Many children, however, who are referred for endocrinologic evaluation are not completely "normal" on physical examination or laboratory testing. Since such children often have undergone or may undergo extensive evaluation, including additional laboratory and radiologic studies, it is beneficial to label their nondisease so that repeated, unnecessary testing can be avoided and parents can be reassured of the child's relative lack of pathology. This is certainly true for the grossly obese child with "non-Cushing syndrome" or "nonpituitary disease," who, nevertheless, requires treatment for obesity. It is also true for children with nonpubertal variants of secondary sexual characteristics, including premature thelarche and adrenarche, and for newborns who exhibit a physiologic surge in TSH secretion or who have TBG deficiency, but not true thyroid disease.
While the list of endocrine nondiseases discussed here is by no means exhaustive, it should serve to alert physicians to the wide variety of such conditions that can occur among children in their practices and help develop criteria for diagnosing them so as to avoid unnecessary anxiety for the children and their parents.
The author would like to acknowledge the critical review of this article by groups of practicing pediatricians in Billings, MT; Colorado Springs, CO; and Grand Junction, CO; and especially by Dr. Jay Markson of Denver.
REFERENCES
1. Park MJ, Kim HS, Kang JH, et al: Serum levels of insulin-like growth factor (IGF)-I, free IGF-I, IGF-binding protein (IGFBP)-1, IGFBP-3, and insulin in obese children. J Pediatr Endocrinol Metab 1999;12:139
2. Srinivasan SR, Myers L, Berenson GS: Temporal association between obesity and hyperinsulinemia in children, adolescents and young adults: The Bogalusa Heart Study. Metabol Clin Exper 1999;48:928
3. Odeleye OE, de Courten M, Pettitt DJ, et al: Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes 1997;46:1341
4. Sotos JF, Cutler ER, Dodge P: Cerebral gigantism. Am J Dis Child 1977;131:625
5. Dodge PR, Kolmes SJ, Sotos JF: Cerebral gigantism. Devel Med Child Neurol 1983;25:248
6. Zonana J, Sotos JF, Romshe CA, et al: Dominant inheritance of cerebral gigantism. J Pediatr 1977;91:251
7. Nydick M, Bustos J, Dale JH, et al: Gynecomastia in adolescent boys. JAMA 1961;178:449
8. Kerem E, Urbach J, Reifen RM, et al: Transient hyperphosphatasemia of infancy. Israel J Med Sci 1987;23:890
9. Garty BZ, Nitzan M: Benign transient hyperphosphatasemia. Israel J Med Sci 1994;30:66
10. Asami T, Gomi T, Uchiyama M: Persistent non-familial asymptomatic hyperphosphatasemia: A report on three cases. Acta Paediatr 1995;84:346
11. Abbassi V, Colon AR, Schwartz RH: Benign elevation of serum alkaline phosphatase, transient and persistent variety. Clin Pediatr 1984;23:336
12. Marx SJ, Attie MF, Levine MA, et al: The hypocalciuric or benign variant of familial hypercalcemia: Clinical and biochemical features in fifteen kindreds. Medicine (Baltimore) 1981;60:397
13. Sargent JD, Stukel TA, Kresel J, et al: Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr 1993;123:393
14. Sidberry GK, Iannone R (eds): Harriet Lane Handbook, ed 15. St. Louis, Mosby, 2000
15. Heath H III: Familial benign hypercalcemia: From clinical description to molecular genetics. West J Med 1994;160:554
16. Lloyd SE, Pannett AA, Dixon PH, et al: Localization of familial benign hypercalcemia, Oklahoma variant (FBHOk) to chromosome 19q13. Am J Hum Genet 1999;64:189
17. Kappy M, Taylor L: Benefits of second screening for congenital hypothyroidism. Abstracts of the International Society for Neonatal Screening, Stockholm, 4th Meeting, 1999, p 63; updated, January 2000
18. Lightner ES: Congenital hypothyroidism: Clues to an early clinical diagnosis. J Fam Pract 1977;5:527
19. Fisher DA: Screening for congenital hypothyroidism. Hosp Pract 1977;12:73
20. Dietz HC, Pyeritz RE: Mutations in the human gene for fibrillin-1 (FBN-1) in the Marfan syndrome and related disorders. Hum Molec Genet 1995;4:1799
21. Maron BJ, Moller JH, Seidman CE, et al: Impact of laboratory molecular diagnosis on contemporary diagnostic criteria for genetically transmitted cardiovascular diseases. Circulation 1998;98:1460
22. De Paepe A, Devereux RB, Dietz HC, et al: Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417
23. Desposito F, Cho S, Frias J, et al: Health supervision for children with Marfan syndrome. Pediatrics 1996;98:978
24. Meador CK: The art and science of nondisease. N Eng J Med 1965;272:92
25. Yager J, Young RT: Nonhypoglycemia is an epidemic condition. N Eng J Med 1974;291:907
THE AUTHOR is Chief of Pediatric Endocrinology at the University of Colorado Health Sciences Center, The Children's Hospital, Denver.
Michael Kappy. Endocrine nondisease: Pituitary and other imposters. Contemporary Pediatrics 2000;11:63.