Erythema Infectiosum in a 7-Year-Old Boy
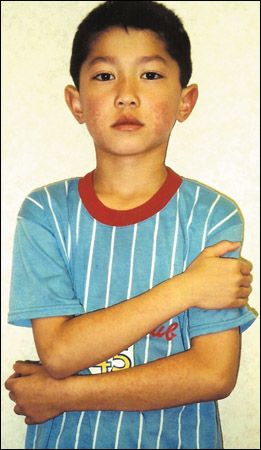
Seven-year-old boy with red, nonpruritic rash that appeared first on the cheeks and then spread to the trunk, extremities, and buttocks. No history of respiratory, GI, or other symptoms in the several weeks before the onset of the rash. Patient is otherwise healthy.
HISTORY
Seven-year-old boy with red, nonpruritic rash that appeared first on the cheeks and then spread to the trunk, extremities, and buttocks. No history of respiratory, GI, or other symptoms in the several weeks before the onset of the rash. Patient is otherwise healthy.
PHYSICAL EXAMINATION
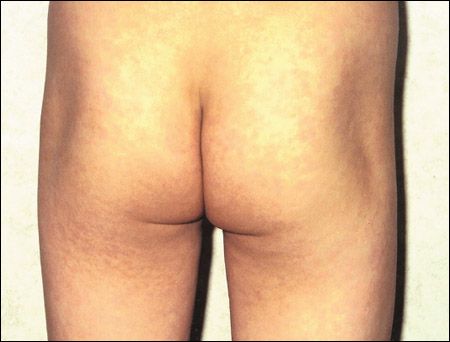
The child is afebrile. A lacy erythematous rash is noted on the trunk, extremities, and buttocks.
Erythema infectiosum-also known as fifth disease-is a benign self-limited exanthematous illness of childhood. The condition was the fifth common childhood exanthem to benamed (after measles, scarlet fever, rubella, and Filatov-Dukes disease [an atypical scarlet fever]).
"WHAT'S YOUR DIAGNOSIS?"
ANSWER: ERYTHEMA INFECTIOSUM (FIFTH DISEASE)
ETIOLOGY
Erythema infectiosum is caused by parvovirus B19-a member of the genus Erythrovirus in the family Parvoviridae. Parvovirus B19 is the only member of the Parvoviridae family known to cause disease in humans.1 The virus was first discovered in 1975.2 The name is derived from the Latin word parvum, which means small. The designation B19 refers to the laboratory number that was used to identify the first positive isolate, which was located in the 19th cell in row B of a panel of sera.
Parvovirus B19 is a nonenveloped, icosahedral, single-stranded DNA virus that is composed of approximately 5600 nucleotides, and is 20 to 25 nm in diameter.3,4 The capsid structure and the absence of an envelope are responsible for the resistance of the virus to heat, cold, and detergents.5 The viral genome encodes 1 nonstructural protein, NS1, and 2 capsid proteins, VPI and VP2. NS1 is involved in the regulation of viral promoter and replication functions and is toxic to the host cells. Four percent of the capsid protein is VP1 and 96% is VP2.5 VP1 and VP2 self-assemble to form viral particles in vitro.3
PATHOGENESIS
After infection, there is a brisk viremia. Parvovirus B19 has an affinity for late erythroid progenitor cells in the bone marrow6 and leads to transient erythroid aplasia. Erythrocyte P blood group antigen-a surface glycolipid present in erythroid cells-is acellular receptor for parvovirus B19. These receptors are also found on endothelial cells and on cells in the placenta, fetal heart, and liver.
The rash appears after the viremia has cleared and coincides with the development of IgG antibody to parvovirus B19.7 Persons who lack the blood group P antigen are immune to parvovirus B19 infection.8
EPIDEMIOLOGY
Infection with parvovirus B19 is reported worldwide and is common. Children between the ages of 6 and 14 years are most often affected.9 Theprevalence of IgG antibodies against parvovirus B19 ranges from 2% to 15% in children under 5 years, 15% to 60% in thoseaged 6 to 19 years, and 30% to 60% in adults; these antibodies are present in more than 85% of the pediatric population.10
Most community outbreaks occur in the winter and spring.5 Parvovirus B19 is spread mainly by respiratory droplets.4 Secondary spread among susceptible school contacts and family members occurs in 20% and50% of cases, respectively.11 Transmission usually occurs during the week before the onset of symptoms. Transmission via percutaneous exposure to parvovirus B19-infected blood or blood products and vertical transmission from a mother to a fetus can also occur.11 The incubation period is 4 to 21 days.12
CLINICAL MANIFESTATIONS
Prodromal symptoms occur in 20% to 60% of cases.13 These are usually mild and consist of low-grade fever, headache, malaise, myalgia, and coryza.14 A symptom-free period of 1 to 7 days usually follows the prodrome andprecedes the exanthem.13 Because symptoms are so mild and temporally separated from the onset of the rash, the prodrome is oftennot recollected.
The rash typically evolves in 3 stages. The initial stage is an erythematous rash on the cheeks, with a characteristic "slapped cheek" appearance. A circumoral pallor is usually present. In the second stage, the rash spreads concurrently or quickly to the trunk, extremities, and buttocks as a diffuse macular erythema.1 The rash tends to be more intense on extensor surfaces. The palms and soles are spared.14 Central clearing of the rash results in the characteristic lacy or reticulated appearance. Pruritus is noted in up to 15% of patients. The rash usually resolves spontaneously within 3 weeks.1
The third stage is characterized by evanescence and recrudescence.15 Triggers for recrudescence include exercise, emotional stress, hot baths, and sunlight.13 The third stage usually lasts for a few weeks, but recurrences may be noted for months.13
COMPLICATIONS

Arthritis and arthralgiacan occur as a complication of erythema infectiosum or as the sole clinical manifestation of parvovirus B19 infection. Although common in adults and older adolescents, arthropathy occurs in only 8% to 10% of children with erythema infectiosum.16 Arthropathy is more common in females than males.1,3 The most commonly affected joints are the metacarpophalanges, followed by the knees, wrists, and ankles.10 Symptoms are usually bilateral.1,16 Thearthropathy might be immunologically mediated because its onset coincides with the appearance of circulating antibodies.10
The arthropathy is self-limited and does not cause destruction of the affected joints. The condition usually resolves in a few weeks.4,5
Transient aplastic crisis, another potential complication, results from an arrest of erythropoiesis and the reticulocytopenia induced by parvovirus B19. It usually lasts for 7 to 10 days.17 The transient reticulocytopenia usually has little impact on an otherwise healthy child. However, children with decreased red cell production are especially prone to a transient symptomatic aplastic crisis.3,13,18 (This includes children with iron deficiency anemia; ongoing blood loss; or a chronic hemolytic anemia, such as hereditary spherocytosis, sickle cell disease, thalassemia, glucose-6-phosphate dehydrogenase deficiency, pyruvate kinase deficiency, or anautoimmune hemolytic anemia.) Persistent parvovirus B19 infection may develop in immunodeficient patients with resultant severe anemia from pure red cell aplasia.3,13
Other less commonly reported hematologic complications following parvovirus B19 infection include transient neutropenia, thrombocytopenic purpura, Henoch-Schönlein purpura, hemophagocytic syndrome, and Diamond-Blackfan syndrome.10,19-22
Other rare complications that have been reported in association with erythema infectiosum or parvovirus B19 infection include aseptic meningitis, encephalitis, brachial plexus neuropathy, carpal tunnel syndrome, Guillain-Barré syndrome, acute cerebellar ataxia, myocarditis, hepatitis, hypocomplementemic post-infectious glomerulonephritis, chronic fatigue syndrome, Gianotti-Crosti syndrome, Kawasaki disease, and papular-purpuric gloves-and-socks syndrome.4-6,23-29
Maternal infection with parvovirus B19 during pregnancy is associated withnonimmune hydrops fetalis, fetal anemia, high output cardiac failure, and spontaneous abortion.30,31 The highest risk is during the first 20 weeks of gestation.32 The risk of fetal loss in the first 20 weeks of gestation is 15%, compared with 5% in controls.33 A congenital infection syndrome in newborn infants has been described and includes anemia, a blueberry-muffin rash, and hepatosplenomegaly.34
DIAGNOSIS/THERAPY
The diagnosis is based on the typical clinical presentation of a "slapped cheek" appearance, with absent or mild prodromal symptoms, followed by a symmetrical, reticulated rash that waxes and wanes. Laboratory diagnosis is rarely necessary.
When laboratory confirmation is required, determination of anti-parvovirus B19 IgM antibody is the preferred diagnostic test in an immunocompetent host. IgM antibody is detectable within 1 to 3 weeks of exposure and is generally present for 2 to 3 months.11 The presence of serum IgG antibody indicates previous infection and immunity, although seroconversion from an IgG-negative to an IgG-positive state suggestsrecent infection.1
In an immunocompromised patient, serologic diagnosis is unreliable. In this setting, demonstration of the virus by nucleic acid hybridization or polymerase chain reaction assay is the optimal diagnostic method.
Differential diagnostic considerations include scarlet fever, rubella, measles, enteroviral infection, drug reaction, and collagen vascular disease.
In immunocompetent patients, there is no specific antiviral therapy and treatment is symptomatic. The rash develops after the viremia has cleared. Because the virus can no longer be transmitted, there is no need to isolate affected children or to restrict attendance at school or child care facilities.
References:
REFERENCES:
1.
Adler SP, Koch WC. Parvovirus infections. In: Gershon AA, Hotez PJ, Katz SL, eds.
Krugman's Infectious Diseases of Children.
11th ed. Philadelphia: Mosby; 2004:429-441.
2.
Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera.
Lancet.
1975;1:72-73.
3.
Brown KE. Human parvovirus B19. In: Long SS, Pickering LK, Prober CG, eds.
Principles and Practice of Pediatric Infectious Diseases.
2nd ed. New York: Churchill Livingstone; 2003:1101-1104.
4.
Young NS, Brown KE. Parvovirus B19.
N Engl J Med.
2004;350:586-597.
5.
Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases.
Curr Opin Infect Dis.
2001;14:343-356.
6.
Huerta-Brogeras M, Izquierdo JA, Hernanz Hermosa JM, et al. Petechial exanthem in "bathing trunk" distribution caused by parvovirus B19 infection.
Pediatr Dermatol.
2005;22:430-433.
7.
Sabella C, Goldfarb J. Parvovirus B19 infections.
Am Fam Physician.
1999;60:1455-1460.
8.
Brown KE, Hibbs JR, Gallinella G, et al. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen).
N Engl J Med.
1994;330: 1192-1196.
9.
Vafaie J, Schwartz RA. Parvovirus B19 infections.
Int J Dermatol.
2004;43: 747-749.
10.
Heegaard ED, Brown KE. Human parvovirus B19.
Clin Microbiol Rev.
2002;15:485-505.
11.
Frydenberg A, Starr M. Slapped cheek disease. How it affects children and pregnant women.
Aust Fam Physician.
2003;32:589-592.
12.
Weir E. Parvovirus B19 infections: fifth disease and more.
CMAJ.
2005;172:743.
13.
Leach CT, Jenson HB. Erythema infectiosum (fifth disease). In: Jenson HB, Baltimore RS, eds.
Pediatric Infectious Diseases. Principles and Practice.
Philadelphia: WB Saunders Company; 2002:325-330.
14.
Leung AK, Kao CP. A collage of infectious diseases in children.
Consultant For Pediatricians.
2004;3:482-487.
15.
Cherry JD. Erythema infectiosum. In: Feigin RD, ed.
Textbook of Pediatric Infectious Diseases.
5th ed. Philadelphia: Saunders; 2004:1799-1809.
16.
Mancini A. Erythema infectiosum (fifth disease). In: Schachner LA, Hansen RC, eds.
Pediatric Dermatology.
3rd ed. New York: Mosby; 2003:1064-1066.
17.
Stiefel L. Erythema infectiosum (fifth disease).
Pediatr Rev.
1995;16:474-475.
18.
Kellermayer R, Faden H, Grossi M. Clinical presentation of parvovirus B19 infection in children with aplastic crisis.
Pediatr Infect Dis J.
2003;22:1100-1101.
19.
Inoue S, Kinra NK, Mukkamala SR, Gordon R. Parvovirus B-19 infection: aplastic crisis, erythema infectiosum and idiopathic thrombocytopenic purpura.
Pediatr Infect Dis J.
1991;10:251-253.
20.
Muir K, Todd WT, Watson WH, Fitzsimons E. Viral-associated haemophagocytosis with parvovirus-B19-related pancytopenia.
Lancet.
1992;339:1139-1140.
21.
Mustafa MM, McClain KL. Diverse hematologic effects of parvovirus B19 infection.
Pediatr Clin North Am.
1996;43:809-821.
22.
Robson WL, Leung AK. Henoch-Schönlein purpura.
Adv Pediatr.
1994;41: 163-194.
23.
Carrascosa JM, Just M, Ribera M, Ferrandiz C. Papular acrodermatitis of childhood related to poxvirus and parvovirus B19 infection.
Cutis.
1998;61: 265-267.
24.
Grilli R, Izquierdo MJ, Farina MC, et al. Papular-purpuric "gloves and socks" syndrome: polymerase chain reaction demonstration of parvovirus B19 DNA in cutaneous lesions and sera.
J Am Acad Dermatol.
1999;41:793-796.
25.
Jacobson SK, Daly JS, Thorne GM, McIntosh K. Chronic parvovirus B19 infection resulting in chronic fatigue syndrome: case history and review.
Clin Infect Dis.
1997;24:1048-1051.
26.
Minohara Y, Koitabashi Y, Kato T, Nakajima N. A case of Guillain-Barré syndrome associated with human parvovirus B19 infection.
J Infect.
1998;36:327-328.
27.
Nigro G, Bastianon V, Colloridi V, Ventriglia F, et al. Human parvovirus B19 infection in infancy associated with acute and chronic lymphocytic myocarditis and high cytokine levels: report of 3 cases and review.
Clin Infect Dis.
2000;1:65-69.
28.
Sokal EM, Melchior M, Cornu C, et al. Acute parvovirus B19 infection associated with fulminant hepatitis of favourable prognosis in young children.
Lancet.
1998;352:1739-1741.
29.
Takeda S, Takaeda C, Takazakura E, Haratake J. Renal involvement induced by human parvovirus B19 infection.
Nephron.
2001;89:280-285.
30.
Kinney JS, Anderson LJ, Farrar J, Strikas RA. Risk of adverse outcomes of pregnancy after human parvovirus B19 infection.
J Infect Dis.
1988;157:663-667.
31.
Xu J, Raff T, Muallem N, Neubert AG. Hydrops fetalis secondary to parvovirus B19 infections.
J Am Board Fam Pract.
2003;16:63-68.
32.
Sohan K, Carroll S, Byrne D, et al. Parvovirus as a differential diagnosis of hydrops fetalis in the first trimester.
Fetal Diagn Ther.
2000;15:234-236.
33.
Miller E, Fairley CK, Cohen BJ, Seng C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy.
Br J Obstet Gynaecol.
1998;105: 174-178.
34.
Silver MM, Hellmann J, Zielenska M, et al. Anemia, blueberry-muffin rash, and hepatomegaly in a newborn infant.
J Pediatr.
1996;128:579-586.