FDA accepts NDA for roflumilast foam 0.3% for seborrheic dermatitis in 9 years and up
Roflumilast is a once-daily, topical phosphodiesterase type 4 (PDE4) inhibitor under development for treatment of inflammatory dermatoses, focusing on the hair-bearing areas including the face, scalp, and trunk.
FDA accepts NDA for roflumilast foam 0.3% for seborrheic dermatitis | Image Credit: © ZayNyi - © ZayNyi - stock.adobe.com
Arcutis Biotherapeutics has announced the FDA has accepted to review the New Drug Application (NDA) for roflumilast foam 0.3% (Arcutis Biotherapeutics) for the potential treatment of seborrheic dermatitis in patients 9 years and older. The agency has set a Prescription Drug User Fee Act (PDUFA) target action date of December 16, 2023.
Roflumilast is a once-daily, topical phosphodiesterase type 4 (PDE4) inhibitor under development for the treatment of inflammatory dermatoses, focusing on the hair-bearing areas including the face, scalp, and trunk.
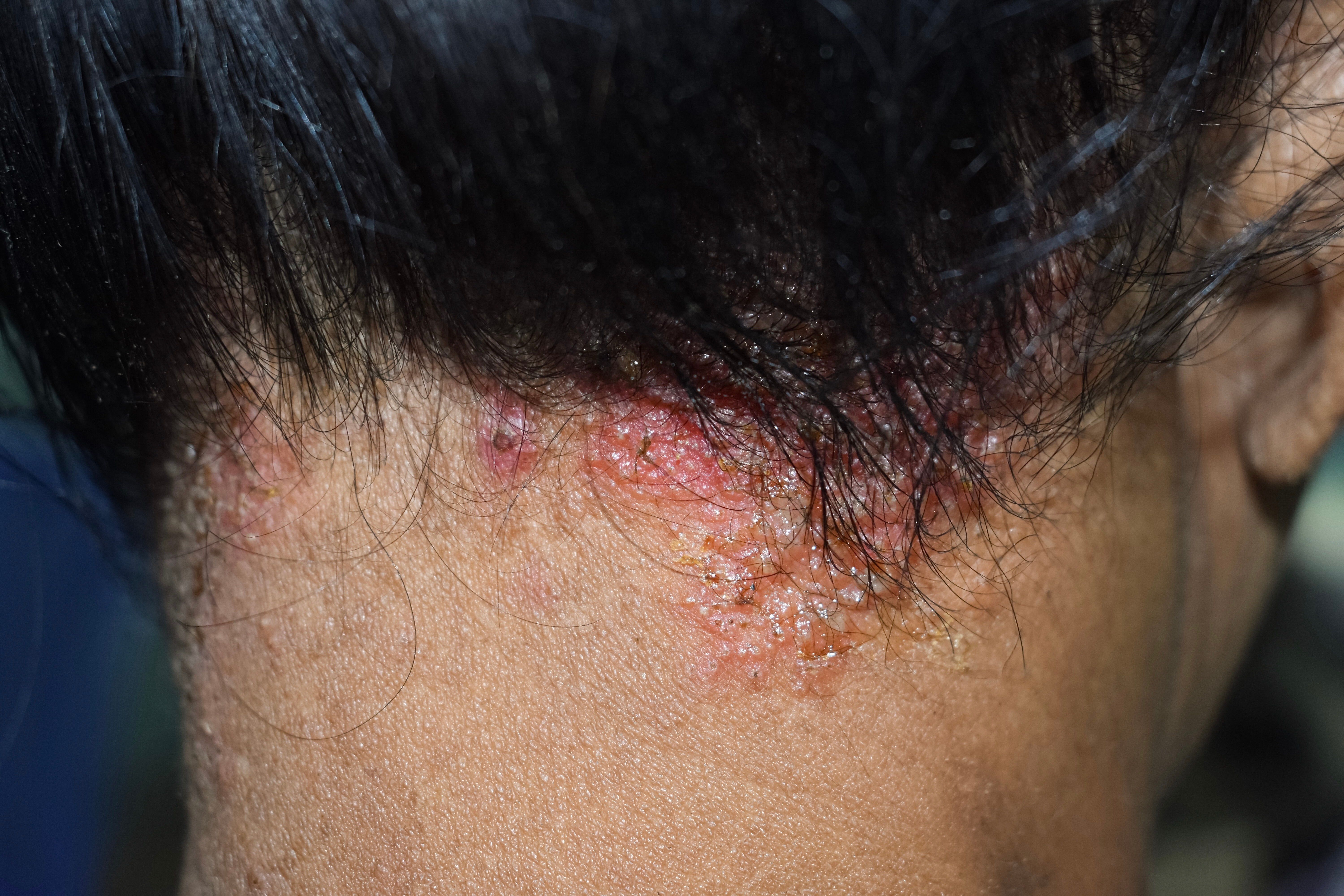
Seborrheic dermatitis affects over 10 million people in the United States and is characterized as a chronic and recurrent inflammatory skin disease consisting of red patches with large, greasy, flaking yellow-gray scales, as well as a persistent itch. Additionally, seborrheic dermatitis often occurs in oil-producing areas of the body such as the scalp, face, upper chest, and back.
“Seborrheic dermatitis has long been a disease in need of its own treatment,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research and an investigator for Arcutis. “Some of the biggest challenges of current treatments have not only been lack of efficacy and consequences from long-term use, but also the limitations that affect adherence, especially the inability to treat both hair- and non-hair-bearing areas. Roflumilast foam was designed to address these shortcomings, as a once-daily, steroid-free topical drug that can be used chronically anywhere on the body. Dermatologists will be excited to incorporate roflumilast foam, if approved, as a new standard of care for those living with seborrheic dermatitis.”
The NDA acceptance is backed by positive data from phase 2 and 3 trials. Specifically, the STudy of Roflumilast foam Applied Topically for the redUction of seborrheic derMatitis (STRATUM) phase 3, parallel-group, double-blind, vehicle-controlled study, the safety and efficacy of roflumilast foam 0.3% as a treatment for seborrheic dermatitis was evaluated.
Results demonstrated the study met its primary endpoint of achieving an Investigator Global Assessment (IGA) Success rate of 79.5% in patients treated with roflumilast vs 58.0% (P < 0.0001) vehicle-treated patients at week 8. Also, investigators observed an early and statistically significant improvement with roflumilast foam compared to vehicle on IGA Success at week 2. Moreover, 51.3% of roflumilast-treated patients achieved complete skin clearance at week 8.
There was also a statically significant improvement over vehicle observed on all secondary endpoints, such as scaling, itch, and erythema. Results demonstrated over 60% of patients reached an itch response at week 8 (62.8% roflumilast foam vs 40.6% vehicle; p = 0.0001), and at weeks 2 and 4, significant improvements in itch were observed.
The study also showed roflumilast foam was well-tolerated, recording a favorable safety and tolerability profile. There was a low incidence of Treatment Emergent Adverse Events (TEAEs) and were similar between roflumilast and vehicle. Also, there were no treatment-related Serious Adverse Events (SAEs).
In the phase 2 and 3 combined studies, more than 90% of roflumilast completed the 8-week treatment period, with few discontinuations due to adverse events (0.9% and 2.2% in the roflumilast foam and vehicle groups, respectively). The most common adverse events were seen in ≥1% of subjects in the combined study populations and included nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast is currently FDA- approved as a 0.3 cream (Zorvye) for the treatment of plaque psoriasis in adults and adolescents.
Reference:
Arcutis Biotherapeutics. Arcutis announces FDA acceptance of new drug application for roflumilast foam 0.3% for the treatment of seborrheic dermatitis in individuals aged 9 years and older. Biospace. April 18, 2023. Accessed April 18, 2023. https://www.biospace.com/article/releases/arcutis-announces-fda-acceptance-of-new-drug-application-for-roflumilast-foam-0-3-percent-for-the-treatment-of-seborrheic-dermatitis-in-individuals-aged-9-years-and-older/
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.