Fluoroquinolone use in children: Resistance and safety implications
Increased use of fluoroquinolones has been associated with some decreased susceptibility to these agents among Streptococcus pneumoniae. Consider the potential for a further increase in the prevalence of antimicrobial resistance, as well as safety concerns in children, when prescribing fluoroquinolones.
Fluoroquinolone use in children:
Resistance and safety implications
By Jeffrey L. Blumer, PhD, MD
Increased use of fluoroquinolones during the past decade has been associated with some decreased susceptibility to these agents among Streptococcus pneumoniae. Pediatricians should consider the potential for a further increase in the prevalence of antimicrobial resistance, as well as safety concerns in children, when prescribing fluoroquinolones.
Nalidixic acid, the first quinolone antimicrobial, has been used in the treatment of urinary tract infections since the 1960s.1 During the past 40 years, newer fluoroquinolones have been developed, with a spectrum of activity that covers both gram-positive and gram-negative pathogens.2 Despite extensive clinical use of the fluoroquinolone antimicrobials in adults for a range of infectious diseases, the data on the pharmacokinetics, efficacy, and safety of fluoroquinolones in children are limited.
Fluoroquinolones have been used in pediatric patients in select cases. Available data in children are largely the result of ciprofloxacin use in select patients with pulmonary infection, such as children with cystic fibrosis. Fluoroquinolones have also been used in cases in which potential benefit was thought to outweigh risk, such as chronic suppurative otitis media, complicated urinary tract infection, uncomplicated gonorrhea, life-threatening salmonellosis and shigellosis, antimicrobial-resistant meningitis, multidrug-resistant tuberculosis, and central nervous system (CNS) infection.3,4 (Topical fluoroquinolones approved for pediatric use are indicated for ocular infections, otitis externa, and acute otitis media in patients with pressure-equalizing tubes.)
This article reviews the published literature describing the low but increasing prevalence of Streptococcus pneumoniae isolates with reduced susceptibility to systemic fluoroquinolones that has been observed in Canada and Hong Kong, and the potential adverse effects of systemic fluoroquinolones that clinicians should consider when prescribing these agents for children.
Antimicrobial resistance
A major concern with expanding the scope of fluoroquinolone therapy to include pediatric indications is the potential problem of increased fluoroquinolone resistance among strains of S pneumoniae, a common community-acquired respiratory pathogen. (See "What causes fluoroquinolone resistance?") Streptococcus pneumoniae is the most commonly reported pathogen in acute otitis media,5 community-acquired pneumonia,6 and sinusitis.7 It is also commonly implicated in meningitis and bacteremia.8 Infections caused by S pneumoniae are responsible for more than 1 million deaths worldwide each year among children younger than 5 years of age.8
Currently, fluoroquinolones are used as last-line treatment in pediatric patients who have failed therapy with other antimicrobials or in those allergic to other classes of antimicrobials. It may be important to preserve the efficacy of fluoroquinolones by thus limiting their use because of the increasing rates of resistance to more commonly used classes of antimicrobialsnamely, ß-lactam and macrolide agents.
Since the first case report of a penicillin-nonsusceptible S pneumoniae isolate in the United States in the mid 1970s,9 resistance among S pneumoniae has continued to increase. A hospital-based pneumococcal surveillance study from 1979 to 1987 reported that isolates of S pneumoniae resistant to penicillin increased from 1.8% in 1979 to 3.6% in 1987.10 By 19941995, results from a national surveillance study demonstrated that the rate of nonsusceptibility to penicillin had jumped to 23.6%.11 Of 1,531 isolates of S pneumoniae collected in the winter of 19992000, 34.2% were not susceptible to penicillin (minimum inhibitory concentration [MIC] >0.12 µg/mL).12 Results from a US surveillance study showed significant cross-resistance between penicillins and macrolides/ azalides among isolates of S pneumoniae, with 66% of penicillin-resistant strains also demonstrating resistance to macrolide/azalide antimicrobials.13
For this reason, as resistance to ß-lactam antimicrobials has increased during recent years, reported rates of macrolide resistance have also jumpedfrom 10.3% in 19941995 to 26.2% by 19992000.12 A study in the US showed a 13% increase in macrolide use between 1993 and 1999, with a 320% increase in macrolide use among children younger than 5 years of age.14 The majority of macrolide prescriptions in this population were for azithromycin: Its use increased by 1.5 million prescriptions overall between 1996 and 1999. Macrolide resistance was found in 30.6% of patients younger than 5 years of age, compared with 16.0% of patients 5 years of age and older (P <.001), the latter being a population in which the total number of macrolide prescriptions did not change during that period. Antimicrobial resistance among strains of S pneumoniae to the many classes of antimicrobials has increased, and this increase may be linked to the exposure of children to new classes of antimicrobials, suggesting a possible weakening of the effectiveness of new agents.
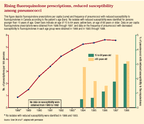
(The figure labled "Rising fluoroquinolone prescriptions, reduced susceptibility among pneumococci," is available in the print edition. Adapted with permission from: Chen DK, McGeer A, DeAzavedo JC, Low DE, for the Canadian Bacterial Surveillance Network: Decreased susceptibility of Streptococcus pneumoniae to fluoroquinoloes in Canada, N Engl J Med 1999;341:233.)
Although fluoroquinolone resistance rates in the US are relatively stable at present,12 there have been several reports of increasing incidence of S pneumoniae with decreased fluoroquinolone susceptibility in adult patients in Canada and Hong Kong in recent years.15,16 Canadian susceptibility data from 7,551 S pneumoniae isolates demonstrated that, although the overall prevalence of S pneumoniae isolates with reduced fluoroquinolone susceptibility during the 10-year period was 1% (75/7,551), the prevalence of reduced fluoroquinolone susceptibility increased from 0% (0/327) in 1988 and 1993, to 1.7% (32/1,844) in 19971998 (P = .01).15 During this same period, the number of fluoroquinolone prescriptions (per 100 persons per year) increased from 0.8 to 5.5 (see figure). The total MIC range of selected fluoroquinolones against 75 ciprofloxacin-resistant S pneumoniae isolates collected from 1994 through 1998 is represented in Table 1 (Adapted with permission in the print issue from Chen DK, McGeer A, DeAzavedo JC, Low DE, for the Canadian Bacterial Surveillance Network: Decreased susceptibility of Streptococcus pneumoniae to fluoroquinoloes in Canada, N Engl J Med 1999;341:233). As ciprofloxacin use increased, along with reported resistance to this agent, decreased susceptibility to other fluoroquinolones manifested as increased MICs for those agents.
PLEASE NOTE: If you are using Internet Explorer version 6 or higher, after clicking to enlarge each graphic in the article, click the picture icon that appears in the lower right-hand corner of the graphic.
Results from a multicenter study in Hong Kong in 2000 demonstrated increasing resistance to levofloxacin compared with results from a 1998 study.16 Of 180 pneumococcal isolates, 13.3% were nonsusceptible (MIC >4 µg/mL) to levofloxacin,16 an increase from 5.5% in 1998.17 Isolates not susceptible to levofloxacin demonstrated cross-resistance to other fluoroquinolones, with median MICs for gatifloxacin and moxifloxacin that were 24- and 32-fold higher, respectively, than for isolates susceptible to levofloxacin.16 These newer agents may not be useful in the treatment of infection caused by levofloxacin-nonsusceptible strains. A recent study demonstrated that treatment with older fluoroquinolones (ciprofloxacin or levofloxacin) may preclude effective treatment with newer agents (moxifloxacin or gatifloxacin).18 In this study, prior therapy with fluoroquinolones was shown to be a risk factor (odds ratio, 10.6; P <.001) for infection with levofloxacin-resistant S pneumoniae.
Fluoroquinolones and adverse events
The safety profiles of fluoroquinolones in adults are well established. Although there are limited safety data in children, the incidence of adverse events with ciprofloxacin in pediatric patients is similar to that seen in adults19; it is, therefore, possible to extrapolate adverse events observed in adults to children. The most commonly reported adverse events associated with the fluoroquinolones for both pediatric and adult patients include gastrointestinal, CNS, dermatologic, and hepatic effects (Table 2, Adapted with permission in the print edition from Fish DN: Fluoroquinolone adverse effects and drug interactions. Pharmacotherapy 2001;21(10 pt 2):253S). The majority of these adverse events are mild and reversible. With regard to the pediatric population, it is the less common adverse events, such as arthropathy and tendon rupture, that are of concern to clinicians when prescribing fluoroquinolones.
Musculoskeletal. Pediatric fluoroquinolone use has been restricted because of the potential for these agents to induce cartilage toxicity in immature animals, including primates, rats, rabbits, mice, and dogs.2025 In these animal models, fluoroquinolone use resulted in localized lesions in the cartilage of weight-bearing joints.26 Nalidixic acid, norfloxacin, ciprofloxacin, difloxacin, and ofloxacin have been associated with such cartilage changes in immature animals.2025 The arthrogenic effects of the fluoroquinolones appear to be species-specific and drug-dependent. For example, 30 mg/kg/day of ciprofloxacin is required to induce cartilage damage in dogs, whereas 500 mg/kg/day is required to produce the same effect in rats.23
The mechanism of fluoroquinolone-induced cartilage toxicity is unclear. It may be caused by inhibition of mitochondrial DNA synthesis within chondrocytes, direct cartilage toxicity with fluoride, or magnesium deficiency in the cartilage from chelation.27 Whatever the mechanism, histologic findings demonstrate blister formation, degeneration of the matrix, and erosion of cartilage. Approximately 1% of patients who are given a fluoroquinolone experience arthropathy, which manifests as joint pain, swelling, or stiffness in weight-bearing joints, such as the knees, elbows, or shoulders.28 Reported incidences of arthralgia associated with ciprofloxacin treatment in pediatric patients range from 1.3% to 3.2%.2931 Note that many of these pediatric patients were given a fluoroquinolone for cystic fibrosis, and as many as 7% of patients with cystic fibrosis experience arthropathy.32 In addition, children with cystic fibrosis have a lower exercise tolerance than children who do not have this disease; this incidence may, therefore, be underrepresented.33
Ten reports of possible fluoroquinolone-induced arthropathy in male and female patients between 14 and 17 years of age have been described in detail. These cases describe joint pain or swelling in the weight-bearing joints (knees, hips, shoulders).27,3441 Joint swelling, pain, or both occurred after treatment with pefloxacin in the majority of these reports (7/10); two patients were treated with ciprofloxacin and one patient received nalidixic acid. However, pefloxacin is not available in the US and similar adverse events have not been reported with levofloxacin, gatifloxacin, or moxifloxacin.
The possibility of tendinitis or tendon rupture is of even greater concern than arthropathy. Although rare (one case for every 175,000 to 800,000 daily doses), these are serious complications of fluoroquinolone use.19 Between 1985 and 1992, 100 cases of fluoroquinolone-induced tendon disorders, including 31 tendon ruptures, were reported in France.42 By 1994, the postmarketing spontaneous reporting system of the US Food and Drug Administration (FDA) had received 25 reports of tendon rupture in patients given a fluoroquinolone, three of which occurred in the US.43 These postmarketing reports of tendinitis and tendon rupture led the FDA to update the labeling on all fluoroquinolones to recommend discontinuation of treatment at the first sign of tendon discomfort or inflammation.
Gastrointestinal. The estimated incidence of gastrointestinal adverse events following fluoroquinolone use is 2% to 20%.28 Most commonly, patients experience nausea, anorexia, or dyspepsia. Abdominal pain, vomiting, and diarrhea also occur and may be more severe. Gatifloxacin and moxifloxacin are associated with the highest incidence of nausea and diarrhea among the fluoroquinolones.
A recent clinical trial evaluated gatifloxacin (10 mg/kg/day) for the treatment of recurrent acute otitis media in 254 children age 6 months to 7 years.44 Safety data were reported for 223 children, and the most common adverse events were vomiting (15%) and diarrhea (5%). The high rate of vomiting may have been a result of the liquid formulation, which was not very palatable. Pediatric clinical trials of ciprofloxacin have reported incidences of gastrointestinal adverse events, including nausea, vomiting, diarrhea, and abdominal pain, ranging from 4.3% to 16.4%.29,4547
Keep in mind that most antimicrobials cause gastrointestinal adverse events in children to a varying degree. The reported incidences of gastrointestinal adverse events in studies using common antimicrobials in pediatric patients are 11.1% (diarrhea) with amoxicillin-clavulanate (Augmentin ES-600),48 7.3% of patients treated with azithromycin (Zithromax) in phase II and phase III studies, and 15.5% of patients treated with clarithromycin (Biaxin) in phase III trials.49
Central nervous system. CNS effects, the second most common adverse event reported in patients receiving fluoroquinolones, occur in 1% to 2% of adult patients, although this can range from 0.2% to 11% for individual agents within this class of antimicrobials.28 The most common symptoms among adult patients are headache, dizziness, and drowsiness. Other disturbances include sleep disorders (such as restlessness and insomnia), agitation, confusion, delirium, psychosis, tremor, seizure, and visual disturbances.28,50 The reported incidence of CNS adverse events with ciprofloxacin therapy among adolescents and children ranges from 0.8% to 4.8%.4547 The potential for CNS adverse events in a pediatric population must be considered because there are many unanswered questions regarding how these effects influence school performance, language skills, and other learning processes.
Dermatologic. Fluoroquinolones cause dermatologic adverse effects in 0.5% to 3% of patients.28,51 In pediatric clinical trials studying ciprofloxacin, dermatologic adverse events, including rash, urticaria, and pruritus, occurred in 2.4% to 15% of patients younger than 18 years.29,4547 Although rare, phototoxic reactions, ranging from mild erythema to bullous eruptions, are more common with ciprofloxacin and levofloxacin than with moxifloxacin or gatifloxacin.27 This reaction is an important consideration for children, who often spend more time outdoors than adults. Less common dermatologic adverse effects include edema, erythema multiforme, hemorrhagic bullae, vasculitis, and dermatitis.45,50
Clinical implications
For the pediatric patient, the fluoroquinolones represent a new class of antimicrobials with a number of inherent advantages. They are bactericidal agents with a broad spectrum of antimicrobial activity, excellent tissue penetration, and availability in both oral and parenteral formulations. As such, they offer a therapeutic alternative to the commonly used antimicrobials in a number of clinical situations for which there have heretofore been limited options (Table 3). These drugs have been used in a number of these clinical scenarios in infants and children, with beneficial effects reported.3,2931,4547,5265 The most commonly used agents have been ciprofloxacin and levofloxacin, both administered as 20 to 30 mg/kg/day in two or three divided doses. More recently, clinical trials have been reported with the 8-methoxyfluoroquinolone gatifloxacin, demonstrating effectiveness and tolerability at a dose of 10 mg/kg administered once a day. Nevertheless, caution persists with regard to the potential for bacteria to develop resistance to these agents, as well as to their safety profile in infants and children.
TABLE 3
When might you consider a fluoroquinolone for a child?
Data suggest that increased use of a given class of antimicrobials leads to increased resistance in that class.15,16 There have been several reports in recent years of growing bacterial resistance to fluoroquinolones, which may be associated with increased fluoroquinolone use. This resistance may continue if widespread fluoroquinolone use extends to the pediatric population.
In terms of the safety of fluoroquinolones in pediatric patients, data are limited. What is needed is a well-designed, prospective, clinical trial of adequate sample size that evaluates the long-term safety of fluoroquinolone use in children. The safety profiles of these agents are well established in adults, however, and may be extrapolated to the pediatric population. Fluoroquinolones are associated with a high incidence of gastrointestinal adverse events, ranging from 2% to 20% depending on the agent. CNS and dermatologic adverse events occur with measurable frequency. The potential for detrimental musculoskeletal effects, including cartilage toxicity in pediatric patients, is of special concern. Isolated cases of fluoroquinolone-induced arthropathy in children have been reported with pefloxacin, an agent unavailable for use in the US, and animal data have demonstrated that fluoroquinolones can induce cartilage damage in juvenile animals.
Serious adverse effects, notably musculoskeletal disturbances, should be a major determining factor when considering fluoroquinolone use in the pediatric population. Exposure to a fluoroquinolone may subject a child to unnecessary risks when safe and effective alternatives are available. Because safety data in this population are limited, the International Society of Chemotherapy recommends the use of alternative effective antimicrobials for pediatric infections.65 Alternatives to fluoroquinolones, such as amoxicillin-clavulanate,5,66,67 cefprozil,68 cefixime,69 cefpodoxime,70 clarithromycin, and azithromycin,49 have a long history of efficacy and safety in children.
Bottom line: Use appropriately and cautiously
Fluoroquinolones should be used prudently in children, who are often colonized with respiratory pathogens such as S pneumoniae. These agents should be reserved for treatment in patients who have failed other antimicrobial therapies or in those who are allergic to ß-lactams or macrolides. Further, it may be best to reserve their use for treatment of severe infections, such as infectious exacerbations of cystic fibrosis or gastrointestinal infections caused by Salmonella or Shigella, for which few alternative antimicrobial agents are available. Pediatricians may feel constrained in their use of these agents even in these situations, because of both the absence of labeling for these drugs in pediatric patients and the warning that appears on some existing labels concerning the development of arthropathy in young animals. Although these concerns are genuine, the off-label use of drugs in pediatric patients is common and often justifiable.71
Widespread use of fluoroquinolone antimicrobials by large groups of pediatric outpatients would open the door to adverse events and may help promote the development of antimicrobial resistanceand, ultimately, may limit the usefulness of these agents. We are fortunate, though: We already have an armamentarium of safe and effective antimicrobials for pediatric patients that precludes the need to introduce fluoroquinolones as first-line treatment of non-life-threatening infection. When conventional therapy fails or cannot be tolerated by a particular patient, however, the potential benefit of fluoroquinolone use may outweigh concerns about safety and antimicrobial resistance. In this way, the fluoroquinolones make an effective addition to our anti-infective armamentarium.
REFERENCES
1. Appelbaum PC, Hunter PA: The fluoroquinolone antibacterials: Past, present, and future perspectives. Int J Antimicrob Agents 2000;16:5
2. Blondeau JM: Expanded activity and utility of the new fluoroquinolones: A review. Clin Ther 1999;21:3
3. Alghasham AA, Nahata MC: Clinical use of fluoroquinolones in children. Ann Pharmacother 2000;34:347
4. Burstein GR, Berman SM, Blumer JL, et al: Ciprofloxacin for the treatment of uncomplicated gonorrhea infection in adolescents: Does the benefit outweigh the risk? Clin Infect Dis 2002;35(suppl 2):S191
5. Dagan R, Hoberman A, Johnson C, et al: Bacteriologic and clinical efficacy of high dose amoxicillin/ clavulanate in children with acute otitis media. Pediatr Infect Dis J 2001;20:829
6. Niederman MS, Mandell LA, Anzueto A, et al: Guidelines for the management of adults with community-acquired pneumonia: Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730
7. Sinus and Allergy Health Partnership: Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2000;123(1 Pt 2):S1
8. Obaro SK, Monteil MA, Henderson DC: The pneumococcal problem. BMJ 1996;312:1521
9. Naraqi S, Kirkpatrick GP, Kabins S: Relapsing pneumococcal meningitis: Isolation of an organism with decreased susceptibility to penicillin G. J Pediatr 1974;85:671
10. Spika JS, Facklam RR, Plikaytis BD, Oxtoby MJ, and the Pneumococcal Surveillance Working Group: Antimicrobial resistance of Streptococcus pneumoniae in the United States, 19791987. J Infect Dis 1991; 163:1273
11. Doern GV, Brueggemann A, Holley HP Jr, et al: Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: Results of a 30-center national surveillance study. Antimicrob Agents Chemother 1996;40:1208
12. Doern GV, Heilmann KP, Huynh HK, et al: Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 19992000, including a comparison of resistance rates since 19941995. Antimicrob Agents Chemother 2001;45:1721
13. Jacobs MR, Bajaksouzian S, Zilles A, et al: Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 US Surveillance Study. Antimicrob Agents Chemother 1999; 43:1901
14. Hyde TB, Gay K, Stephens DS, et al, for the Active Bacterial Core Surveillance/Emerging Infections Program Network: Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA 2001; 286:1857
15. Chen DK, McGeer A, DeAzavedo JC, Low DE, for the Canadian Bacterial Surveillance Network: Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med 1999;341:233
16. Ho PL, Yung RWH, Tsang DNC, et al: Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: Results of a Hong Kong multicentre study in 2000. J Antimicrob Chemother 2001;48:659
17. Ho PL, Que TL, Tsang DN, et al: Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother 1999;43:1310
18. Ho PL, Tse WS, Tsang WT, et al: Risk factors for acquisition of levofloxacin-resistant Streptococcus pneumoniae: A case-control study. Clin Infect Dis 2001;32:701
19. Ball P, Mandell L, Niki Y, et al: Comparative tolerability of the newer fluoroquinolone antibacterials. Drug Saf 1999;21:407
20. Burkhardt JE, Hill MA, Carlton WW, et al: Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol 1990;27:162
21. Gough A, Barsoum NJ, Mitchell L, et al: Juvenile canine drug-induced arthropathy: Clinicopathological studies on articular lesions caused by oxolinic and pipemidic acids. Toxicol Appl Pharmacol 1979;51:177
22. Kato M, Onodera T: Morphological investigation of cavity formation in articular cartilage induced by ofloxacin in rats. Fundam Appl Toxicol 1988;11:110
23. Linseman DA, Hampton LA, Branstetter DG: Quinolone-induced arthropathy in the neonatal mouse: Morphological analysis of articular lesions produced by pipemidic acid and ciprofloxacin. Fundam Appl Toxicol 1995;28:59
24. Machida M, Kusajima H, Aijima H, et al: Toxicokinetic study of norfloxacin-induced arthropathy in juvenile animals. Toxicol Appl Pharmacol 1990;105:403
25. Stahlmann R, Merker HJ, Hinz N, et al: Ofloxacin in juvenile non-human primates and rats: Arthropathia and drug plasma concentrations. Arch Toxicol 1990;64:193
26. Hayem G, Carbon C: A reappraisal of quinolone tolerability: The experience of their musculoskeletal adverse effects. Drug Saf 1995;13:338
27. Burkhardt JE, Walterspiel JN, Schaad UB: Quinolone arthropathy in animals versus children. Clin Infect Dis 1997;25:1196
28. Fish DN: Fluoroquinolone adverse effects and drug interactions. Pharmacotherapy 2001;21(10 pt 2):253S
29. Chysky V, Kapila K, Hullmann R, et al: Quinolones in clinical use. Safety of ciprofloxacin in children: Worldwide clinical experience based on compassionate useemphasis on joint evaluation. Infection 1991;19:289
30. Hampel B, Hullman R, Schmidt H: Ciprofloxacin in pediatrics: Worldwide clinical experience based on compassionate usesafety report. Pediatr Infect Dis J 1997;16:127
31. Kubin R: Safety and efficacy of ciprofloxacin in paediatric patients: Review. Infection 1993;21:413
32. Phillips BM, David TJ: Pathogenesis and management of arthropathy in cystic fibrosis. J R Soc Med 1986;79(suppl 12):44
33. de Meer K, Gulmans AM, van der Laag J: Peripheral muscle weakness and exercise capacity in children with cystic fibrosis. Am J Respir Crit Care Med 1999;159:748
34. Alfaham M, Holt M, Goodchild M: Arthropathy in a patient with cystic fibrosis taking ciprofloxacin. Br Med J 1987;295:699
35. Chang H, Chung M-H, Kim JH, et al: Pefloxacin-induced arthropathy in an adolescent with brain abscess. Scand J Infect Dis 1996;28:641
36. Chevalier X, Albengres E, Voisin MC, et al: A case of destructive polyarthropathy in a 17-year-old youth following pefloxacin treatment. Drug Saf 1992;7:310
37. Jawad ASM: Cystic fibrosis and drug-induced arthropathy. Br J Rheumatol 1989;28:179
38. Kesseler A, Lacassie A, Hugot JP, et al: Arthropathies following the administration of pefloxacin to an adolescent with mucoviscidosis. Ann Pediatr 1989;36:275
39. Le Loët X, Fessard C, Noblet C, et al: Severe polyarthropathy in an adolescent treated with pefloxacin (letter). J Rheumatol 1991;18:1941
40. Ollier S, Laroche M, Arlet P, et al: Arthropathy caused by pefloxacin: Report of a case. Rev Rheum Mal Osteoartic 1990;57:671
41. Seigneuric C, Plantavid M, Bouygues D, et al: Joint manifestations of pefloxacin in adolescents. Presse Med 1990;19:428
42. Pierfitte C, Gillet P, Royer RJ: More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med 1995;332:193
43. Szarfman A, Chen M, Blum M: More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med 1995;332:193
44. Arguedas A, Sher L, Lopez E, et al: Gatifloxacin (GAT) treatment of recurrent/non-responsive acute otitis media (RNR-AOM). Presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept. 2225, 2001; Chicago, Ill.
45. Church DA, Kanga JF, Kuhn RJ, et al: Sequential ciprofloxacin therapy in pediatric cystic fibrosis: Comparative study vs. ceftazidime/tobramycin in the treatment of acute pulmonary exacerbations. Pediatr Infect Dis J 1997;16:97
46. Richard DA, Nousia-Arvanitakis S, Sollich V, et al: Oral ciprofloxacin vs. intravenous ceftazidime plus tobramycin in pediatric cystic fibrosis patients: Comparison of antipseudomonas efficacy and assessment of safety with ultrasonography and magnetic resonance imaging. Pediatr Infect Dis J 1997;16:572
47. Schaad UB, Wedgwood J, Ruedeberg A, et al: Ciprofloxacin as antipseudomonal treatment in patients with cystic fibrosis. Pediatr Infect Dis J 1997;16:106
48. Augmentin ES-600 prescribing information. Research Triange Park, N.C.: GlaxoSmithKline; 2002
49. Klein JO: History of macrolide use in pediatrics. Pediatr Infect Dis J 1997;16:527
50. Lipsky BA, Baker CA: Fluoroquinolone toxicity profiles: A review focusing on newer agents. Clin Infect Dis 1999;28:352
51. Ball P, Tillotson G: Tolerability of fluoroquinolone antibiotics. Drug Saf 1995;13:343
52. Dagan R, Schlaeffer F, Einhorn M: Parenteral fluoroquinolones in children with life-threatening infections. Infection 1990;18:237
53. Freifeld A, Pizzo P: Use of fluoroquinolones for empirical management of febrile neutropenia in pediatric cancer patients. Pediatr Infect Dis J 1997;16:140
54. Gbadoe AD, Dogba A, Dagnra AY, et al: Acute osteomyelitis in the child with sickle cell disease in a tropical zone: Value of oral fluoroquinolones. Arch Pediatr 2001;8:1305
55. Gendrel D, Moulin F: Fluoroquinolones in paediatrics. Paediatric Drugs 2001;3:365
56. Hoffman MA, Diamond D: Do fluoroquinolones have a role in pediatric urinary tract infections? Infect Med 2000;17:334
57. Lang R, Goshen S, Raas-Rothschild A, et al: Oral ciprofloxacin in the management of chronic suppurative otitis media without cholesteatoma in children: Preliminary experiences in 21 children. Pediatr Infect Dis J 1992;1 1:925
58. Mullen CA, Petropoulos D, Roberts WM, et al: Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 1999;86:126
59. Patrick CC: Use of fluoroquinolones as prophylactic agents in patients with neutropenia. Pediatr Infect Dis J 1997;16:135
60. Patrick CC, Freifeld A, Green S, et al: Panel discussion: Ciprofloxacin/quinolone use in non-cystic fibrosis patients. Pediatr Infect Dis J 1997;16:160
61. Redmond AO: Risk-benefit experience of ciprofloxacin use in pediatric patients in the United Kingdom. Pediatr Infect Dis J 1997;16:147
62. Redmond A, Sweeney L, MacFarland M, et al: Oral ciprofloxacin in the treatment of pseudomonas exacerbations of paediatric cystic fibrosis: Clinical efficacy and safety evaluation using magnetic resonance image scanning. J Int Med Res 1998;26:304
63. Rubio TT: Ciprofloxacin in the treatment of Pseudomonas infection in children with cystic fibrosis. Diagn Microbiol Infect Dis 1990;13:153
64. Rubio TT, Miles MV, Lettieri JT, et al: Pharmacokinetic disposition of sequential intravenous/oral ciprofloxacin in pediatric cystic fibrosis patients with acute pulmonary exacerbation. Pediatr Infect Dis J 1997;16:112
65. Schaad UB, Salam MA, Aujard Y, et al: Use of fluoroquinolones in pediatrics: Consensus report of an International Society of Chemotherapy commission. Pediatr Infect Dis J 1995;14:1
66. Damrikarnlert L, Jauregui AC, Kzadri M: Efficacy and safety of amoxycillin/clavulanate (Augmentin) twice daily versus three times daily in the treatment of acute otitis media in children. The Augmentin 454 Study Group. J Chemother 2000;12:79
67. Subba Rao SD, Macias MP, Dillman CA, et al: A randomized, observer-blind trial of amoxycillin/clavulanate versus cefaclor in the treatment of children with acute otitis media. Augmentin 415 Study Group. J Chemother 1998;10:460
68. Wilber RB, Doyle CA, Durham SJ, et al: Safety profile of cefprozil. Clin Infect Dis 1992;14(suppl 2):S264
69. Kafetzis DA: Multi-investigator evaluation of the efficacy and safety of cefprozil, amoxicillin-clavulanate, cefixime, and cefaclor in the treatment of acute otitis media. Eur J Clin Microbiol Infect Dis 1994;13:857
70. Cohen R: Clinical experience with cefpodoxime proxetil in acute otitis media. Pediatr Infect Dis J 1995;14(suppl 4):S12
71. Blumer JL: Off-label uses of drugs in children. Pediatrics 1999;104(3 Pt 2):598
DR. BLUMER is chief, pediatric pharmacology and critical care medicine, Rainbow Babies and Children's Hospital, Cleveland, Ohio. He receives grant/research support from, is a consultant for, and is a member of the speakers' bureau of Bristol-Myers Squibb, GlaxoSmithKline, Bayer, and Johnson & Johnson. He received editorial assistance in, and payment for, creating the manuscript of this article from Scientific Therapeutics Information, Inc, on behalf of GlaxoSmithKline.
What causes fluoroquinolone resistance?
Fluoroquinolones act by targeting two bacterial enzymes involved in DNA replication: DNA gyrase and topoisomerase IV.1 Resistance to fluoroquinolones increases when bacterial genes undergo mutations, specifically the gyrA and gyrB genes of gram-negative bacteria and the parC or parE of gram-positive organisms.2 The first-step mutation, which affects the primary drug target, slightly decreases antimicrobial susceptibility. When mutations occur in additional targets, susceptibility is reduced further.1 Mutations in these genes, especially mutations at two or more targets, can reduce susceptibility to all available fluoroquinolones.
In addition to genetic mutations, fluoroquinolone resistance can result from expression of efflux mechanisms within bacteria that pump antimicrobial agents out of the cell. Piddock and colleagues3 reported that the presence of an efflux pump among S pneumoniae decreases the in vitro activity of the fluoroquinolones, including the newer agents moxifloxacin and gatifloxacin. These mechanisms have been implicated in levofloxacin treatment failures.47 Treatment failures have not been reported with gatifloxacin or moxifloxacin.
REFERENCES
1. Hooper DC: Mechanisms of action and resistance of older and newer fluoroquinolones. Clin Infect Dis 2000;31(suppl 2):S24
2. Hooper DC: Mechanisms of action of antimicrobials: Focus on fluoroquinolones. Clin Infect Dis 2001;32 (suppl 1):S9
3. Piddock LJV, Johnson M, Ricci V, et al: Activities of new fluoroquinolones against fluoroquinolone-resistant pathogens of the lower respiratory tract. Antimicrob Agents Chemother 1998;42:2956
4. Davidson R, Cavalcanti R, Brunton JL, et al: Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med 2002;36:747
5. Empey PE, Jennings HR, Thornton AC, et al: Levofloxacin failure in a patient with pneumococcal pneumonia. Ann Pharmacother 2001;35:687
6. Kays MB, Smith DW, Wack ME, et al: Levofloxacin treatment failure in a patient with fluoroquinolone-resistant Streptococcus pneumoniae pneumonia. Pharmacotherapy 2002;22:395
7. Urban C, Rahman N, Zhao X, et al: Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. J Infect Dis 2001;184:794
Jeffrey Blumer. Fluoroquinolone use in children: Resistance and safety implications. Contemporary Pediatrics November 2003;20:97.