A guide to abscesses in the skin
Pediatricians can handle most cutaneous and subcutaneous abscesses. The site of the lesion, agent of infection, and age and immunologic status of the child are the keys to treatment strategies.
A guide to abscesses in the skin
Gary L. Darmstadt, MD
Pediatricians can handle most cutaneous and subcutaneousabscesses. The site of the lesion, agent of infection, and age and immunologicstatus of the child are the keys to treatment strategies.
Abscesses in the skin tend to look the same wherever they are on thebody. The site of the abscess, along with the age and immunologic statusof the patient, determines how the abscess is managed, however. The generalpediatrician who is familiar with typical presentations can handle mostabscesses, though in certain situations he or she may want to enlist theassistance of dermatology, immunology, infectious disease, or surgical colleagues.
The abscesses discussed here include furuncles and carbuncles, abscessesof the apocrine glands and the areas around the nail or rectum, and thebreast and scalp abscesses found in the neonate. Before reviewing how todiagnose and treat these abscesses, I will explain what qualities definean abscess and why they develop.
What abscesses are and where they come from
An abscess is a localized collection of pus in a cavity, formed whentissue disintegrates or necrotizes. Clinically, an abscess is a firm, tender,erythematous nodule that becomes fluctuant. The child with an abscess generallyhas no constitutional symptoms unless the process has extended into deepertissues or the bloodstream. An abscess of the skin is covered by normalepidermis, but the dermis contains a dense aggregate of acute inflammatorycells surrounded by a fibrinoid wall.
Predisposing factors. Cutaneous and subcutaneous abscesses most oftendevelop when a primary infection extends locally into the epidermis or dermis,for example when an abscess originates from a skin appendage, such as afuruncle, carbuncle, epidermal inclusion cyst, or, rarely, periporitis (astaphylococcal infection complicating miliaria). Abscesses also arise whena secondary infection develops at a site of skin disease or injury. Traumathat allows pathogens to invade subcutaneous tissue can lead to an abscess,as can the spread of pathogens through the blood, though this is uncommon.Table 1lists predisposing factors for development of abscesses. Often, nosuch factor can be identified in the child with an abscess.

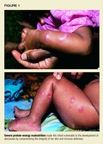
Processes that disrupt the integrity of the skin, such as severe malnutrition,may pave the way for abscesses (Figure 1). A deficiency in local or systemicimmunologic defense, particularly of neutrophils, also can lead to abscesses.Conditions that cause such a deficiency include congenital or acquired neutropenia,leukocyte adhesion deficiency, and chronic granulomatous disease.1Abscesses are particularly likely to accompany hyperIgE syndrome, whichincludes defective neutrophil chemotaxis, and leukocyte adhesion deficiency.2Patients with chronic granulomatous disease are less likely to have skininfections than children with neutrophil migration defects, but they seemmore prone to invasive infections of deep tissues, the bloodstream, or solidorgans.
Pediatricians should suspect immunodeficiency, and consider a referral,when a child has:
- Recurrent abscesses
- Unusually severe infection with a common organism such as Staphylococcus aureus or Candida albicans
- An abscess caused by an organism not normally associated with abscesses, especially one of low virulence
- Complications, such as bacteremia or septicemia.
Although many immunodeficiency diseases are associated with abscesses,mostchildren with recurrent abscesses are not immunodeficient.
Causative organisms.S aureus is the most common pathogen in cutaneousand subcutaneous abscesses (Table 2). It is particularly prevalent, frequentlyas the sole pathogen, in abscesses of the neck, trunk, and extremities,though it can be found in all areas where abscesses originate on skin surfaces(Figure 2).3 S aureus alsois the culprit in the vast majorityof abscesses from which only one organism is isolated, suggesting that bacterialsynergism is not important in the pathogenesis of these lesions.3,4Skin infections in patients with defective neutrophil migration are usuallycaused by S aureus,Calbicans, or gram-negative bacilli. In chronic granulomatousdisease, the most common agents of cutaneous infection are S aureus, gram-negativebacilli, and fungi, such as Aspergillis.
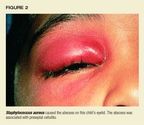

The principal pathogens of abscesses vary with the location of the lesion,however. Most abscesses of the perineal region (inguinal, buttocks, perirectal,and vulvovaginal) contain many anaerobic stool organisms, particularly Bacteroides,although anaerobes occasionally appear to act alone to cause the abscess.
Cultures from perineal or perioral abscesses and ulcers typically containorganisms that originated from adjacent mucous membranes rather than skin,whereas lesions far from the rectum or mouth contain organisms that normallyreside on skin.4
In general, the causative organism(s) should be identified whenever aneonate or an immunocompromised individual has an abscess. Identificationalso is called for when the abscess is recurrent or recalcitrant to standardtherapy, requires surgical treatment, or is unusually severe and associatedwith complications such as local or systemic extension of infection. Onoccasion, empiric use of penicillinase-resistant antistaphylococcal antibioticsmay suffice for a first episode in a healthy child with an uncomplicatedsuperficial abscess. But given the recent rise in antibiotic resistanceamong some bacterial pathogens, including those recovered from skin infections,this is not a good routine practice.5
Furuncle and carbuncle
A furuncle, commonly known as a boil, is a cutaneous abscess that developsaround a hair follicle (Figure 3). A carbuncle is a cluster of furuncles.
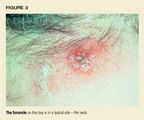
Epidemiology and etiology. These abscesses may or may not be precededby folliculitis. The most common sites are hair-bearing areas on the face,neck, axillae, buttocks, and groin. Furuncles are almost always caused byS aureus, though other bacteria or fungi occasionally may be culprits. Staphhas a predilection for binding to abraded skin around the follicle. Certainstrains of S aureus, such as type 80/81, appear to have a propensity forcausing furuncles.
Predisposing conditions include a warm, humid environment, obesity, excessivesweating, maceration, friction, and preexisting dermatitis. Furuncles aremore common in boys than girls and in individuals with low serum iron, diabetesmellitus, malnutrition, or HIV infection or other immunodeficient states.Most individuals with furunculosis, including those with recurrent disease,do not have an identifiable immune deficiency, however, so the conditiongenerally can be managed without subspecialty referral. Recurrent furunculosisfrequently is associated with carriage of S aureus in the nares, axillae,or perineum, or sustained close contact with a carrier.
Clinical characteristics. A furuncle is a deep-seated, tender, erythematous,perifollicular papule that evolves into a nodule. Influx of neutrophils,followed by vessel thrombosis, suppuration, and central tissue necrosis,leads to rupture and discharge of a central core of necrotic tissue, destructionof the follicle, and scarring. Surrounding cellulitis may develop. Painmay be intense if the lesion is situated where the skin is relatively fixed,such as in the external auditory canal or over nasal cartilage. Patientswith furuncles usually have no constitutional symptoms, though they maydevelop bacteremia, particularly in association with malnutrition or manipulationof the lesion. Rarely, severe lesions on the upper lip or cheek may leadto life-threatening cavernous sinus thrombosis.
A carbuncle is an infection of a group of contiguous follicles, withmany drainage points, and inflammatory changes in surrounding connectivetissue. Carbuncles may be accompanied by fever, leukocytosis, and bacteremia.
Diagnosis. Furunculosis or carbunculosis usually can be diagnosed byclinical inspection.
Treatment. Frequent application of a hot, moist compress facilitatesdrainage of lesions. Large lesions may require surgical drainage, whichgenerally is best accomplished after the lesion has softened and becomefluctuant. Treat carbuncles and large or multiple furuncles, particularlythose located in the center of the face, with oral penicillinase-resistantsystemic antibiotics such as cephalexin, dicloxacillin, or cloxacillin.The penicillin-allergic patient can be treated with clindamycin or erythromycin,though erythromycin resistance among isolates of S aureus limits this antibiotic'susefulness. In the past, recurrent cases were treated successfully by colonizingthe individual with a nonvirulent strain of S aureus such as 502A aftera course of systemic therapy to eradicate the carrier state; this treatmentis no longer practiced, however.
Evaluate a child with recurrent or recalcitrant disease for carriageof S aureus. Approximately three fourths of the time the site of carriageis the nostrils, which can be cultured with a cotton swab; occasionally,the perineum or axillae may be colonized. Applying mupirocin cream witha cotton swab just inside both nares three times daily for five days temporarilyeliminates carriage, though recolonization can be expected in weeks to months.Another way to eliminate carriage is by giving rifampin in combination witha b-lactamase-stable antistaphylococcal antibiotic; do not use rifampinalone because of the risk of selecting for resistant organisms.5Attention to personal hygiene and use of an antibacterial soap also maybe beneficial. If recurrent disease persists, consult an infectious diseaseor dermatology colleague about prophylactic use of a low-dose oral antistaphylococcalpenicillin or clindamycin.6
Hidradenitis suppurativa
This is a chronic, inflammatory, suppurative disorder of the apocrineglands.
Epidemiology and etiology. Hidradenitis suppurativa probably is initiatedwhen the apocrine gland ducts become plugged with keratinous debris (Figure4). Bacterial infection, particularly with S aureus, Streptococcus milleri,Escherichia coli, and possibly anaerobic streptococci, appears to be importantin the progressive dilatation below the obstruction. Hidradenitis suppurativaseems to be androgen dependent, though authorities do not agree about theunderlying mechanism. Onset is usually during puberty or early adulthood,after the apocrine glands are mature. It is three times more common in femalesthan males and more often strikes blacks than whites.
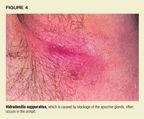
Clinical characteristics. Solitary or multiple painful, erythematousnodules, deep abscesses, and contracted scars are sharply confined to areasof skin containing apocrine glands in the axillae and anogenital region;occasionally, the location is the scalp, behind the ears, on the femalebreasts, or around the umbilicus. When the disease is severe and chronic,sinus tracts, ulcers, and thick, linear fibrotic bands develop.
Hidradenitis suppurativa tends to persist for many years, punctuatedby relapses and partial remissions. Complications include cellulitis, ulceration,and burrowing abscesses, which may perforate adjacent structures in theanogenital region, forming fistulae to the urethra, bladder, rectum, orperitoneum. Some patients develop episodic inflammatory arthritis and afew have the follicular occlusion triad, which includes acne conglobata(a severe chronic form of acne) and perifolliculitis capitis (dissectingcellulitis of the scalp) along with hidradenitis; a tetrad with pilonidalsinus also has been described. These conditions may also occur singly.
Diagnosis. Early lesions are often mistaken for infected epidermal cysts,furuncles, scrofuloderma, actinomycosis, cat-scratch disease, granulomainguinale, or lymphogranuloma venereum. Sharp localization to areas of thebody with apocrine glands should suggest hidradenitis, however. When onlythe anogenital region is involved, the condition may be difficult to distinguishfrom Crohn's disease, with which it may coexist.
Treatment. Patients should avoid tight-fitting clothes, which may exacerbatethe condition. Topical antibiotics such as clindamycin may be sufficient,but during the acute phase of disease I prefer systemic antibiotics chosenon the basis of bacterial culture and susceptibility tests. Empiric therapywithout culture, though less desirable, may be attempted using tetracycline,doxycycline, or minocycline, provided the patient is older than 8 years;clindamycin or cephalosporins also may be effective. Some patients requirelong-term treatment with tetracycline or erythromycin. Intralesional triamcinoloneacetonide (510 mg/mL) is often helpful in early disease. Adding oralprednisone to the regimen of patients who respond poorly to antibioticsmay decrease fibrosis and scarring. The dose should be 40 to 60 mg/day forseven to 10 days, tapering gradually as inflammation subsides.
Since hidradenitis tends to be chronic, recurrent, and associated withscarring or complications or manifestations in other sites, early referralto a dermatologist is recommended. Ultimately, control or cure may requiresurgical measures.
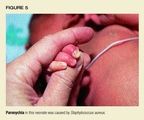
Paronychia
Acute paronychia is a localized inflammation and infection of the areaaround the nail (Figure 5), usually after an injury. The primary disorderis separation of the cuticle from the nail, followed by invasion of thespace by pathogens.
Epidemiology and etiology. Paronychia is most common in children whosuck their fingers or bite their nails, have poor hygiene, or engage inactivities that soak or traumatize the skin around the nail. Usually, mixedoropharyngeal flora are found: S aureus and Steptocococcus pyogenes arethe most common aerobic organisms, while anaerobic pathogens include Bacteroides,Fusobacterium nucleatum, and gram-positive cocci, particularly Peptostreptococcus.7Occasionally, gram-negative organisms such as Pseudomonas, Proteus, andE coli are found. C albicans is implicated in chronic infection.
Clinical characteristics. The lateral nail fold becomes warm, red, edematous,and painful. A purulent exudate may develop. Dermatitis often erupts aroundthe affected area and may contribute to the problem.
Diagnosis. Acute paronychia usually can be diagnosed by clinical inspectionthough it can be confused with herpes simplex virus (HSV) infection, suchas herpetic whitlow, which also occurs in the area around the nail. Herpesinfection typically follows a prodrome of pain and presents as vesicleson an erythematous base. The herpetic lesion may become purulent or necrotic.Because of the thickness of the stratum corneum on the fingers, the vesiclesmay appear more deeply set than is usual with herpes simplex virus infections.It occasionally may be necessary to perform a Tzanck smear or a direct fluorescentantibody test or culture to identify the virus.
Treatment. Attention must be directed toward eliminating the conditionsor activities causing maceration and trauma. Warm compresses three to fourtimes daily generally cure superficial lesions. A collection of pus callsfor drainage. This can be facilitated by gently pushing the surroundingskin away from the nail plate. Use an 18-gauge needle or #11 blade insertedbetween the proximal nail fold and the nail plate.
Treat deep lesions with antibiotics as well as with incision and drainage.Dicloxacillin, cloxacillin, and cephalexin are the antibiotics of choicefor treatment of infections known to be caused by S aureus; amoxicillinplus clavulanic acid is preferred for empiric treatment because of the possibilityof b-lactamase-producing anaerobes.Continue antibiotics until the infectionhas cleared clinically. Chronic paronychia sometimes precedes acute infectionand persists after it has cleared, requiring management.
Perirectal abscess
This abscess is seen in healthy neonates and infants, as well as in immunosuppressedchildren.
Epidemiology and etiology. Perirectal abscess is more common in boysthan girls. No predisposing factor may be apparent, or the abscess may developfollowing minor abrasions or fissures, particularly in association withdiarrhea or constipation. Usually, however, children who develop perirectalabscess have a predisposing condition (Table 3). Perirectal abscess appearsto be initiated by a break in the mucosal barrier or occlusion of anal crypts.Consequently,in children with granulocytopenia, the risk for development of an abscessor perirectal cellulitis increases with perirectal mucositis, hemorrhoids,rectal fissure, or manipulation.

The organisms in perirectal abscesses are predominantly mixed anaerobicand aerobic flora of the intestine and skin of the anal verge, includingS aureus and S pyogenes as well as Bacteroides, Peptococcus, Peptostreptococcus,Porphyromonas, Fusobacterium, Clostridium, E coli, P aeruginosa, Klebsiella,Proteus, and enterococci.810 Rarely, perirectal abscessis due to Entamoeba histolytica, Mycobacterium, Nocardia, or Actinomyces.
Clinical characteristics. A superficial abscess usually causes signsof pain on defecation, sitting, or walking, and redness, swelling, and tendernessin the perianal region. Such an abscess can extend in several directions:downward along the anal sphincter to exit next to the anus on the buttock(fistula-in-ano); to the side through the external sphincter to the ischiorectalfossa to form a deep abscess; or upward to the deep space between the internalsphincter and the levator ani muscles.11 An abscess in deepertissues may be accompanied by poorly localized deep pain and constitutionalsigns. An anorectal abscess may not be apparent externally, but rectal examinationis generally painful. Complications, which are most common in children withunderlying disease, include anorectal fistula, recurrent abscesses, bacteremia,and necrotizing fasciitis.
Diagnosis. A superficial abscess can be diagnosed by clinical inspection,though a deeper abscess may require careful rectal examination. Obtain aculture from a spontaneously draining abscess or surgically and use theresults of susceptibility testing to guide antimicrobial therapy.
Treatment. In immunocompetent infants, a superficial perianal abscessmay drain spontaneously and be self-limiting. It's still a good idea todrain the abscess promptly and explore it and the fistulae. Sitz baths arebeneficial. Antibiotics such as clindamycin and an aminoglycoside to coverS aureus,b-lactamase-resistant anaerobic bacteria, and aerobic gram-negativebacilli may prevent regional spread of the infection and decrease complications.In the absence of fluctuance, extensive soft tissue disease, or sepsis,prescribe a seven to 10-day course of parenteral antimicrobial therapy.If disease progresses or the abscess becomes fluctuant, the child shouldundergo surgery. A chronic fistula may require a fistulotomy.11
In children with granulocytopenia, development of erythema, induration,and fluctuance may be delayed or absent. Early aggressive medical managementwith broad-spectrum antibiotics that cover S aureus, gram-negative bacilli,enterococci, and anaerobes may make surgical intervention unnecessary. Repeatedexamination should be avoided, but close follow-up is critical.
Breast abscess
An abscess in the breast, or in the scalp, develops exclusively in theneonate, while a rectal abscess also may be found in older infants.
Epidemiology and etiology. Breast abscesses are usually caused by S aureus,but the culprit occasionally is group B streptococcus, E coli, Salmonella,Proteus mirabilis, or Pseudomonas aeruginosa.12 Although anaerobicorganisms can be isolated from up to 40% of infections, their pathogenicrole in neonates is questionable and therapy directed specifically againstthem generally is unnecessary.13
Breast abscess develops in full-term neonates during the first six weeksof life, most often the second or third week; premature infants rarely havethis disorder. Although boys and girls are equally likely to develop a breastabscess during the first two weeks of life, girls are twice as likely asboys to have such an abscess thereafter.14 This suggests thatbreast development may be a factor in the pathogenesis of breast abscesssince physiologic breast enlargement is more common in infant girls thanboys after, but not before, 2 weeks of age. The absence of breast abscessin the underdeveloped breast of the premature infant supports this hypothesis.Breast manipulation also may be a predisposing factor.14
In infants with S aureus breast abscess, the nasal or pharyngeal mucousmembranes usually are colonized with the same organism.13S aureusmay spread from the nasopharynx to the skin of the nipple and move fromthere up the ducts of the physiologically enlarged, predisposed breast toinfect deeper tissues.
Clinical characteristics. Breast abscess presents initially with breastenlargement, accompanied by varying degrees of erythema, firmness, and tenderness.Whether the abscess progresses to fluctuancedepends in part on how earlyantibiotic therapy is initiated. Bilateral infection occurs in fewer than5% of cases.13 Only one third of affected infants have feverand most don't have constitutional symptoms; half to two thirds of patientshave leukocytosis (>15,000/mL). In one quarter to one half of patientsbreast abscess caused by S aureus is accompanied by cutaneous pustules orbullae on the trunk, particularly in the perineal region.13,14
Infants with breast abscess caused by gram negative or anaerobic bacillihave similar symptoms, ages, and clinical findings to those infected withS aureus. Infants infected with Salmonella, however,generally also havegastrointestinal illness.12,13 The most common complication ofbreast abscess is cellulitis, which develops in approximately 5% to 10%of affected infants.13,14 Usually it is localized, but it canextend rapidly to the shoulder or abdomen. Other complications such as bacteremia,pneumonia, osteomyelitis, and sepsis are unusual. Scar formation leadingto decreased breast size following puberty can be a late complication.14
Diagnosis. Gram stain of material expressed from the nipple or obtainedby needle aspiration or incision and drainage guides initial antibiotictherapy. The presence of cutaneous vesicles or bullae may help identifyS aureus. Blood cultures should be obtained, but unless the infant has afever or looks ill, cultures of urine and cerebrospinal fluid are unnecessary.
Treatment. If fluctuance is present, the abscess must be drained by needleaspiration, by gently expressing pus from the nipple, or surgically; antibiotictherapy is adjunctive. If fluctuance is absent, early antibiotic therapymay be curative. A b-lactamase-resistant antistaphylococcal antibiotic shouldbe given parenterally.
If gram-negative bacilli are seen on Gram stain or the infant appearsill, initial therapy should include an aminoglycoside or cefotaxime. Ifno organisms are seen on Gram stain, start antibiotics for control of bothS aureus (nafcillin) and gram-negative bacilli (aminoglycoside or cefotaxime)while awaiting culture results. Once infection has begun to subside andconstitutional signs are absent, therapy may be completed orally with cloxacillin,dicloxacillin, or cephalexin if the infection was caused by S aureus alone.In most instances, a total of five to seven days of therapy is sufficient,though many experts continue treatment for 10 to 14 days.13
Scalp abscess
Scalp abscess develops in neonates at the insertion site of a fetal scalpmonitoring electrode.
Epidemiology and etiology. Reported incidence of scalp abscess followingplacement of a spiral fetal scalp electrode, the type used since the early1970s, ranges from 0.1% to 1% in retrospective studies.15 Prospectivestudies reportan incidence of 0.56% and 4.5%.16,17
Predisposing factors have not been clearly identified. The most plausiblehypothesis is that the infection is initiated in utero when normal cervicalflora ascend into the uterus after the membranes rupture, aided by proceduresthat access the uterine cavity. The scalp electrode breaks the cutaneousbarrier and acts as a foreign body, becoming a focus for infection in thesubcutaneous tissue. Risk factors may include long duration of rupturedmembranes and monitoring; monitoring for high-risk indications, particularlyprematurity; and being the mother's firstborn.17,18 Durationof ruptured membranes or of monitoring has not been consistently identifiedas a risk factor, however.16
The number of vaginal examinations, concurrent monitoring with an intrauterinepressure catheter, use of more than one spiral electrode, fetal scalp-bloodsampling, maternal diabetes, and endomyometritis may be associated withscalp abscess.16 Although procedures that increase access ofvaginal flora to the infant or produce more trauma to the scalp may increasethe risk of abscess development, scalp trauma and compression per se arequestionable factors in scalp abscess pathogenesis.15,17
Scalp abscess is typically caused by several microbes. Cultures fromapproximately one third of abscesses reveal aerobic or facultative organismsalone, 10% to 25% grow anaerobes alone, and 40% to 60% grow a mixture ofaerobic or facultative and anaerobic bacteria.15,17,19 The mostcommon aerobic isolates are S aureus, group A, B, and D streptococci, Staphylococcusepidermidis,and occasionally Haemophilus influenzae type b, E coli,Klebsiella pneumoniae,Enterobacter,P aeruginosa, and Neisseriagonorrhoeae. Common anaerobic isolatesare Peptococcus,Peptostreptococcus, Bacteroides,Proprionobacteriumacnes,and Clostridium. The anaerobic flora in the abscesses are those found inthe normal cervix during labor.20
Clinical characteristics. Abscesses most often appear on the third orfourth days of life, but may arise as early as the first day and as lateas three weeks.15 At first thelesion is a localized, hard, redarea 0.5 to 2 cm in diameter. The site may become fluctuant or pustular.Regional lymphadenopathy may be present, but more serious complicationssuch as cranial osteomyelitis and bacteremia are rare.
Diagnosis. During the first weeks of life, closely monitor infants whoundergo scalp electrode monitoring in utero, and instruct their parentsto observe them carefully. If an abscess is noted, remove the hair directlyaround the lesion to allow for closer inspection. Needle aspiration or aswab of the exudate from the puncture site can be used to culture for aerobicand anaerobic organisms. The primary concern is to rule out HSV infection.15Lesions in this disease peak four to 10 days after birth--at the same timescalp abscesses may become apparent--and the two types of lesions may beclinically indistinguishable. If HSV infection seems a possibility, therapywith acyclovir is warranted while awaiting diagnostic test results, sinceHSV lesions may disseminate.
Treatment. Many lesions resolve spontaneously, but fluctuance withouta spontaneous discharge of pus calls for incision and drainage, withoutextensive debridement. If there is cellulitis in the surrounding area, afive- to seven-day course of parenteral antibiotic therapy is usually sufficient;culture results should guide the choice of antibiotic.
Secondary infection of cutaneous tumors
Abscesses occasionally develop in a cutaneous tumor that has become infected,distorting the primary tumor and making it difficult to identify. When thenature of the underlying lesion is in question, consultation with a dermatologistmay facilitate diagnosis and management.
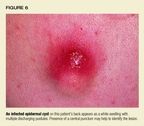
Epidermal cyst. This sharply circumscribed, dome-shaped, skin-coloredcyst is firm and freely movable. It usually appears after puberty. A centraldimple or punctum, an important clue to diagnosis, may signal the locationof a plugged, dilated pore. A mass of layered, keratinized material, perhapswith a cheesy consistency, fills the cavity of the cyst. Epidermal cystsare most common on the face, neck, chest, and upper back and may periodicallybecome inflamed and infected, particularly if the patient has acne vulgaris(Figure 6). The most common causative organism is S aureus. The cyst wallmay rupture, inducing an inflammatory reaction in the dermis and a celluliticappearance.
Excise, drain, and pack a fluctuant, infected cyst, and place the patienton an antibiotic that covers against S aureus. After inflammation subsides,consider removing the cyst.
Pilar(trichilemmal) cyst. This tumoris clinically indistinguishable froman epidermal cyst but is histopathologically distinct and less common. Itappears after puberty as a smooth, firm, mobile nodule, typically on thescalp (90% of cases) and occasionally on the face, neck, or trunk. The cystmay become infected with S aureus, inducing inflammation and occasionallysuppuration and ulceration.
Treat patients with an infected pilar cyst with an antistaphylococcal,b-lactamase-stable oral antibiotic. Once the inflammation has subsided,the cyst can be shelled out easily from the dermis, unlike an epidermalcyst. Inheritance of a propensity to develop pilar cysts is autosomal dominant;these patients usually have more than one cyst. Suspect Gardner's syndromein the patient who has many pilar and epidermoid cysts, desmoid tumors,fibromas, lipomas, or osteomas. Epidermal cysts in Gardner's syndrome tendto be limited to the face and scalp. The syndrome also is associated withcolonic polyposis or adenocarcinoma.
Pilomatricoma.A benign tumor, pilomatricoma is a 3 to 30 mm, firm, solitary,deep dermal or subcutaneous tumor on the head, neck, or upper extremities.It most often develops during the first or second decade of life. Althoughthe overlying epidermis is usually normal, the tumor occasionally is closeenough to the surface to give the overlying skin a blue-red color. Pilomatricomasmay enlarge rapidly because of infection and inflammation or hemorrhageand perforate the epidermis. Infected lesions can be treated in the samemanner as pilar cysts.
Boiling it down
Abscesses usually are diagnosed clinically and treated with antibiotics,though nonpharmacologic management, including drainage and compresses, oftenis helpful. Pediatricians can manage most abscesses but will want to referthe child who requires surgery or may be immunodeficient.
THE AUTHOR is Assistant Professor, Departments of Pediatrics and Medicine,University of Washington School of Medicine, Seattle. He is also AdjunctAssistant Professor, Department of International Health, School of Hygieneand Public Health, Johns Hopkins Medical Institutions, Baltimore, MD.
REFERENCES
1. Darmstadt GL, Marcy SM: Skin and soft tissue infection, in Long SS,Prober CG, Pickering LK (eds): Principles andPractice of Pediatric InfectiousDisease. New York, NY, Churchill Livingstone, 1996
2. Tosi MF: Disorders of phagocyte function, in Patrick CC (ed): Infectionsin Immunocompromised Infants and Children. New York, NY, Churchill Livingstone,1992
3. Brook I, Finegold SM: Aerobic and anaerobic bacteriology of cutaneousabscesses in children. Pediatrics 1981;67:891
4. Ghoneim ATM, McGoldrick J, Blick PWH, et al: Aerobic and anaerobicbacteriology of subcutaneous abscesses.Br J Surg 1981;68:498
5. Darmstadt GL: Oral antibiotic therapy for uncomplicated bacterialskin infections in children. Pediatr Infect Dis J 1997;16:227
6. Klempner MS, Styrt B: Prevention of recurrent staphylococcal skininfections with low-dose oral clindamycin therapy. JAMA 1988;260:2682
7. Brook I: Bacteriologic study of paronychia in children. Am J Surg1981;141:703
8. Brook I, Martin WJ: Aerobic and anaerobic bacteriology of perirectalabscess in children. Pediatrics 1980;66:282
9. Arditi M, Yoger R: Perirectal abscess in infants and children. Reportof 52 cases and review of the literature. Rev Infect Dis 1990;9:411
10. Glenn J, Cotton D, Westley R,et al: Anorectal infections in patientswith malignant disease. Rev Infect Dis 1988;10:42
11. Longo WE, Touloukian RJ, Seashore JN: Fistula in ano in infants andchildren: Implications and management. Pediatrics 1991;87:737
12. Brook I: The aerobic and anaerobic microbiology of neonatal breastabscess. Pediatr Infect Dis J 1991;10:785
13. Walsh M, McIntosh K: Neonatal mastitis. Clin Pediatr 1986;25:395
14. Rudoy RC, Nelson JD: Breast abscess during the neonatal period. AmJ Dis Child 1975;129:1031
15. Cordero L, Anderson CW, Zuspan FP: Scalp abscess: A benign and infrequentcomplication of fetal monitoring. Am J Obstet Gynecol 1983;146:126
16. Wagener MM, Rycheck RR, Yee RB, et al: Septic dermatitis of the neonatalscalp and maternal endomyometritis with intrapartum internal fetal monitoring.Pediatrics 1984;74:81
17. Okada DM, Chow AW, Bruce VT: Neonatal scalp abscess and fetal monitoring:Factors associated with infection. Am J Obstet Gynecol 1977;129:185
18. Plavidal FJ, Werch A: Fetal scalp abscess secondary to intrauterinemonitoring. Am J Obstet Gynecol 1976:125:65
19. Brook I, Frazier EH: Microbiology of scalp abscess in newborns. PediatrInfect Dis J 1992;11:766
20. Thadepalli H, Chan WH, Maidman JE, et al: Microflora of the cervixduring normal labor and the puerperium. J Infect Dis 1978;137:468