Heading off the dangers of acute gastroenteritis
Acute gastroenteritis has many possible causes but two main treatments--oral rehydration and early refeeding. Despite their proved effectiveness in preventing life-threatening dehydration from diarrhea, they are still underused.
Heading off the dangers of acute gastroenteritis
By James Berman, MD
Acute gastroenteritis has many possible causes but two main treatmentsoral rehydration and early refeeding. Despite their proven effectiveness in preventing life-threatening dehydration from diarrhea, they are still underused.
Despite many advances in treatment, acute gastroenteritis remains the leading cause of death for children worldwide. In developing countries, children have an average of 2.2 to 3.3 episodes of diarrhea a year (Table 1) with an annual tally of deaths of 3 to 4 million.1 This means that 1% to 4% of the 1 billion episodes of diarrhea worldwide each year are fatal. In the United States and Canada, mortality from acute diarrhea is estimated to be 325 to 425 deaths a year.2
TABLE 1
Diarrhea in children worldwide Incidence and deaths
Diarrhea also causes significant morbidity. A recent survey in the US noted 49.4 hospitalizations for every 10,000 children annually.3,4 The cost of these hospitalizations alone adds $12 per child to the expense of caring for all children in the US. Physician visits, outpatient care, and lost income from parental absences from work increase the cost substantially.
How pathogens cause diarrhea
Diarrhea can be defined as an increase in the frequency, volume, or liquidity of bowel movements. The mechanism by which infective organisms cause diarrhea varies, and one organism can produce disease by more than one mechanism. Viruses generally injure the absorptive surface of mature villous cells, resulting in decreased fluid absorption and disaccharidase deficiency. Bacteria produce intestinal injury by direct invasion of the mucosa, damage to the villous surface, or elucidation of toxins. All sources of diarrhea can cause significant fluid and electrolyte losses with resultant hypovolemia or acidosis.
Fortunately, the cells that line the gastrointestinal tract can turn over very rapidly. New enterocytes mature into villous surface cells, and most infectious diarrhea begins to resolve within 72 hours.
Acute diarrhea from nongastrointestinal sources, often called parenteral diarrhea, can be associated with infections outside the GI tract, particularly urinary tract infections, otitis media, and pneumonia. Diarrhea also may occur in immunocompromised patients and patients with an inherited defect of the GI tract, a neoplasm, or an endocrinopathy. Acute diarrhea that does not result from infection of the GI tract and chronic diarrhea are beyond the scope of this review.
The usual suspects: Viruses
The causes of acute diarrhea in children vary with location, time of year, and population studied. Viruses are the most common culprits in developed countries.
Rotavirus has been observed consistently to cause at least half of all of cases of acute gastroenteritis, independent of geography. Rotavirus has a distinctive seasonal variation in temperate climates, where cases usually peak in the winter months, packing pediatricians' waiting rooms with sick children. Children between 4 and 24 months of age are most often infected.
The typical rotavirus illness begins after a one- to three-day incubation period with acute onset of fever and vomiting. Watery diarrhea occurs at the onset of vomiting or shortly thereafter. Loose stools usually resolve in three to four days. Dehydration and electrolyte disturbance are the major sequelae of rotavirus infection and occur most often in the youngest children. Rotavirus is responsible for 6% of all deaths in children younger than 5 years.5 In the US, rotavirus results in an emergency room visit or hospitalization for one in 20 children during their first five years.6
Adenoviruses 40 and 41 are the second leading cause of diarrhea. The clinical picture is similar to that of rotavirus. In contrast to the nonenteric adenoviruses, high fevers and respiratory symptoms are rare. Serotype 41 is associated more often with diarrhea in older children, produces more abdominal pain, and has a longer course than serotype 40.
Other viral pathogens that have been observed in the stools of children with diarrhea include pararotavirus and members of the calicivirus family. Pararotavirus resembles its better known cousin rotavirus clinically and morphologically but does not share common antigens.
Small viruses of the calicivirus family have been associated with community outbreaks of diarrhea. They generally have place names like Norwalk, denoting where an outbreak was first observed. The real incidence of diarrhea from these viruses is unknown, but they appear to infect adults more often than young children. Nausea and vomiting are particularly common with calicivirus infections.
Most viral pathogens have proven difficult to grow in tissue culture or detect with enzyme immunoassay techniques. We are left with morphologic descriptions of their appearance under the electron microscope.
Bacterial causes of diarrhea
In developed countries, bacterial pathogens account for 2% to 10% of cases of diarrhea, and parasites for an additional 1% to 8% of cases. The clinical presentation of bacterial gastroenteritis overlaps with viral disease, and the two are most often indistinguishable clinically. A few clinical and laboratory clues make a bacterial cause more likely, however (Table 2). High fevers, shaking chills, and blood in the stools are all unusual in acute viral gastroenteritis. The presence of leukocytes in a stool specimen is helpful for discriminating viral from bacterial gastroenteritis. White blood cells in the stool often predict bacterial gastroenteritis.
TABLE 2
Clinical clues to bacterial gastroenteritis
Campylobacter is the second most common bacterial pathogen isolated, after Salmonella.7 It has a wide clinical spectrum, ranging from mild diarrhea to dysentery. Most cases are self-limited and do not require antibiotic therapy. More severe cases can be treated with erythromycin or ciprofloxacin. Treatment generally improves the clinical course of the disease.
Treating Salmonella, on the other hand, has not been shown to change the natural course of the disease. For that reason, antibiotic therapy should be reserved for patients at risk of Salmonella bacteremia, including infants and patients who are immunocompromised. Because amoxicillin- resistant Salmonella is common, either cefotaxime or ceftriaxone is the drug of choice.
Shigella can produce severe symptoms with a small inoculum of bacteria. High spiking fevers and bloody stools are common. As a toxin producer, Shigella is specifically associated with neurologic symptoms such as seizures with fever. Treatment moderates the course of the disease, making antibiotic therapy worthwhile. The drug of choice for shigellosis is trimethoprim-sulfamethoxazole. Resistance to antibiotics is common. Antimicrobial susceptibility testing of isolates is indicated. Resistant strains may require treatment with ciprofloxacin after careful exploration of the risks and benefits associated with this drug.
Various Escherichia coli subspecies can cause diarrhea. Enteroadherent and enterotoxigenic strains generally produce mild, self-limited loose stools. The latter has ruined many a vacation. Antibiotics and bismuth salicylate shorten the duration of the disease. Antibiotic prophylaxis is not recommended for traveler's diarrhea because the risk of adverse effects, such as photosensitization, outweighs the benefits. Many clinicians, however, suggest beginning antibiotic therapy at the first diarrheal stool. This may achieve a return to a happy vacation more quickly. Trimethoprim-sulfamethoxazole is the most commonly recommended antibiotic.
Enterohemorrhagic E coli is better known by its serotype, O157:H7. Most patients with O157:H7 have mild, uncomplicated diarrhea. Bloody diarrhea and abdominal cramping are common. A more severe dysenteric presentation is also possible. Approximately 5% to 10% of affected children develop hemolytic-uremic syndrome (HUS).8 Because good evidence suggests that previous antibiotic therapy is a risk factor for developing HUS, patients with unexplained bloody diarrhea and those who are O157:H7-positive should not be treated empirically with antibiotics.
Vibrio bacteria, including V cholerae, "non-01" cholera, Aeromonas, and Pleisiomonas, are unusual sources of diarrhea in developed countries.
Parasitic infections
The most common parasite that causes diarrhea in the US is Giardia lamblia. Giardia is a flagellate protozoan that has a worldwide distribution. Most human disease is caused by infection from other humans by the fecal-oral route. Outbreaks also can result from contaminated water supplies or infected animals. Many young children are asymptomatic carriers of Giardia cysts.
Most symptomatic patients have mild diarrhea. Abdominal bloating and cramping are also common. GI bleeding is rare. Giardia is best detected by enzyme immunoassay (EIA) examination of the stools. Direct microscopic identification of cysts or trophozoites in the stool requires three separate stool samples to reach 95% sensitivity.
Metronidazole is the drug of choice for treating symptomatic giardiasis. It must be compounded into a suspension for administration to young children. Furazolidone is an alternative that is supplied in a liquid form. Asymptomatic carriers should not be treated except to prevent spread in situations in which they are in close contact with immunocompromised patients.
Cryptosporidium infection has occurred both as isolated cases and outbreaks associated with contaminated municipal water supplies. It generally produces a benign, self-limited diarrhea, although prolonged loose stools in otherwise healthy children have been seen. Patients who are immunodeficient may experience severe, sometimes secretory, diarrhea and wasting. Supportive care with rehydration and adequate nutrition is usually all that is required. Effective pharmacologic therapy for cryptosporidiosis has not been established.
Entamoeba histolytica (amebiasis) is a common cause of diarrhea and dysentery in developing countries. Most cases in the US have been associated with immigration or travel. Intestinal infection results in one to three weeks of progressive diarrhea, usually with blood. Weight loss and fever occur in more than one third of patients. The chronic nature of this infection and its systemic symptoms may be confused with inflammatory bowel disease. Liver involvement can occur with or without the gastroenteritis.
Extraintestinal involvement may include, in addition to the liver, the lungs, brain, pericardium, skin, and urinary tract. Stool examination demonstrates trophozoites or cysts. E histolytica trophozoites cannot be distinguished morphologically from the more common and nonpathogenic Entamoeba dispar. Serologic testing is more specific and is usually positive in cases of amebic dysentery or liver disease.
Prevention: The best treatment
Prevention remains the most vital measure in managing diarrheal disease. Although developed countries have advanced public health systems to protect the water supply and food chain, gaps in protection still occur. A large municipal water supply in Milwaukee, Wis., became contaminated with Cryptosporidia in 1993,9 and the public has received a steady stream of news reports about meat supplies contaminated with E coli or Listeria. Chickens and eggs have a high rate of bacterial contamination.
Anticipatory guidance can help prevent infection. Parents should be warned to cook ground meat and chicken thoroughly (no pink color should be visible) and to cook eggs so that yolks are not runny. Cutting boards and hands should be washed thoroughly after handling raw meat and poultry. Children infected with rotavirus, E coli O157:H7, or Shigella should be excluded from day care until the diarrhea resolves. In the case of the latter two organisms, two subsequent stool cultures also should be negative before a return to day care.
Oral rehydration therapy
Current treatment of acute gastroenteritis rests on the twin pillars of oral rehydration and early reintroduction of feedings (see "Steps to managing acute gastroenteritis"). Oral rehydration therapy for dehydration caused by diarrhea has a long history of basic science and clinical observation supporting its safety and efficacy.
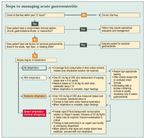
PLEASE NOTE: If you are using Internet Explorer version 6 or higher, after clicking to enlarge the graphic, click the picture icon that appears in the lower right-hand corner of the graphic.
The basic physiology of intestinal absorption is worth reviewing in this context. The intestinal villous cells, which are lost in the course of intestinal injury during a diarrheal illness, are responsible for absorption of luminal contents. Secretion of electrolytes occurs in the crypt cells. Water follows the osmotic gradient associated with these secretions and malabsorbed luminal contents. Sodium ions are actively pumped out of the enterocyte at the apical surface, creating an intracellular sodium gradient. Sodium is absorbed from the intestinal lumen by cotransport with other small molecules and driven by the established sodium gradient. The most common cotransporter of sodium is glucose. This means that glucose must be present in the intestinal lumen to facilitate sodium absorption. This absorptive capacity exists even in the face of severe gastroenteritis, making effective oral rehydration possible.
Treatment of dehydration with the World Health Organization oral rehydration solution (ORS), which contains 90 mmol/L sodium and 20 g/L glucose, has substantially decreased deaths from cholera and other diarrheal diseases. In the US, the absence of cholera, generally high level of nutrition, and generous total body sodium levels in children have led to development of a consensus ORS containing 50 mmol/L sodium. Both solutions have been shown to be safe and effective in treating dehydration associated with acute diarrhea.
Hydration status can be assessed on the basis of easily observable signs and symptoms (Table 3). Children who are not thirsty, have moist mucous membranes, wet diapers, and tears, are not dehydrated and do not require ORS. In the absence of dehydration, ORS should be used to replace ongoing stool losses only in severe casescases in which the patient has already required rehydration and still has ongoing diarrhea.
TABLE 3
Assessing the degree of dehydration
At the other extreme, children who are dehydrated severely enough to cause changes in vital signs or mental status require emergency intravenous fluid resuscitation. Hypotension is a late manifestation of shock in children. Mental status, heart rate, and perfusion are better indicators of severe dehydration and incipient shock. After initial treatment with IV fluids, these children can be given oral rehydration.
Children who are mildly or moderately dehydrated should receive 50 to 100 mL/kg of ORS over four hours and should be reevaluated often for changes in hydration status. It may be necessary to do this under medical supervision. These patients also may receive 10 mL/kg of ORS to replace ongoing losses.
Children who are vomiting generally tolerate ORS. Administering the rehydration solution through a nasogastric tube is possible, but generally unnecessary. Giving ORS, either orally or nasogastrically, is contraindicated in the child who is obtunded or at risk of aspiration. When oral rehydration therapy is complete, regular feedings should be resumed.
Resume feeding early
Early refeeding is recommended in managing acute gastroenteritis because luminal contents are a known growth factor for enterocytes and help facilitate mucosal repair following injury. Introducing a regular diet within a few hours of rehydration, or continuing the diet during diarrhea without dehydration, has been shown to shorten the duration of disease. Early refeeding has not been associated with increased morbidity, such as electrolyte disturbance or a need for IV therapy.
Almost all infants with acute gastroenteritis can tolerate breastfeeding. For formula-fed infants, diluted formula does not provide any benefit over full-strength formula. Infants with more severe diarrhea may require a lactose-free formula until mucosal recovery is complete at around two weeks.
Older children can consume a sensible diet. No data suggest that a diet consisting of only bananas, rice, applesauce, and toast (the BRAT diet) is any more helpful than a regular, age-appropriate diet. Although lactose restriction may not be necessary, most clinicians recommend it. The parent guide ("When your child has diarrhea") provides instructions on home treatment of the child who has diarrhea.
Medication: Not the first choice
The helpful effects of medication as adjunctive therapy for diarrhea are outweighed by the tendency of medications to distract families from the mainstays of oral rehydration and early refeeding. Improved stool consistency or decreased stool frequency produced by medications may lead parents to a false sense of security about true stool fluid losses. Medications are not recommended, therefore, in treating acute diarrhea.
Drugs used to treat diarrhea include loperamide, bismuth subsalicylate, adsorbent agents, and anticholinergic agents. Loperamide is an opioid analog with specificity for intestinal receptors. It decreases stool output and time to first formed stool. Cases of ileus, particularly in infants, raise concerns about its safety. Bismuth subsalicylate can also be effective in acute diarrhea. Its exact mechanism of action is unknown, but it is principally an adsorbent. No reports of Reye syndrome have been associated with the use of bismuth subsalicylate, but a theoretical risk exists. Adsorbent agents, such as attapulgite, have shown effectiveness in acute diarrhea, but data is limited. Anticholinergic agents, such as hyoscyamine, are commonly prescribed in acute gastroenteritis to reduce abdominal cramping. Little data is available on safety or efficacy.
Medications containing diphenoxylate hydrochloride and atropine sulfate, such as Lomotil, have opiate, antimotility, and antisecretory effects. They have been associated with significant toxicity in children and are therefore contraindicated.
Why is effective therapy underused?
The establishment of evidence-based treatment guidelines has not resulted in universal use of the two primary diarrhea treatments. In one survey, fewer than 3% of US pediatricians recommended rapid rehydration (over a span of less than six hours).10,11 Only 42% suggested early reintroduction of feedings. Many did not recommend the standard oral rehydration solution and used other "clear liquids" instead. Many of these solutions varied widely from the standard ORS. European pediatricians showed similar differences in practice from the consensus guidelines.
If oral rehydration and refeeding are scientifically validated, recommended by expert consensus, safe, effective, and inexpensive, why are they so underused? The answers lie in cultural attitudes that work against parental acceptance of oral rehydration therapy and refeeding.
Many parents in developed countries are unwilling to accept that a low-tech, inexpensive solution taken by mouth can be effective. All their experience tells them that an expensive drug, administrated IV in the hospital, is the accepted method of dealing with a severe, potentially life-threatening condition.
Many parents, and some physicians, believe that Western youngsters are too spoiled by highly flavored beverages to drink the bland, slightly salty ORS. However, studies of US children have shown that thirst is the major driver of consumption of ORS and that most children who won't drink the solution are not dehydrated in the first place.
The use of ORS as a "clear liquid" in many clinical situations apart from dehydration no doubt adds to the cultural confusion. Pedialyte, a standard ORS, is given to children who have stomachache and vomiting without diarrhea as well as to postsurgical patients and others. Even in the child with both vomiting and diarrhea, ORS is well tolerated and effective.
Some physicians worry that giving oral rehydration therapy precludes a valid admission to the hospital in the eyes of third-party payers (insurance companies or HMOs). Although there are egregious exceptions, most payers will allow an admission for rehydration without IV therapy if the patient requires substantial observation or nursing support. In such cases, the medical record should document that the parents are exhausted, a trial of outpatient therapy failed, or the child requires frequent measurement of vital signs (every two hours) or nursing reassessments of hydration (every one or two hours). Many hospitals have allocated outpatient or observation beds to help address this issue.
Technical objections to the use of ORS, including the risk of hypernatremic dehydration, have not been borne out by clinical experience. Many older clinicians see oral rehydration as a return to the days before intravenous therapy was widely practiced or appropriately used in children. The difference, of course, has to do with the specific efficacy of ORS, as opposed to other liquids, for providing adequate rehydration. Appropriate IV fluid therapy is still vitally important in managing children with shock associated with dehydration, as well as a wide range of pediatric conditions unrelated to acute diarrhea.
Ways to improve diarrhea management
Decreasing morbidity from diarrhea can be accomplished in several ways (Table 4). With the exception of patient education, more evidence about safety and efficacy is required before recommending these potential advances in treatment.
TABLE 4
Present and future methods of improving the treatment of diarrhea
Patient education. Altering cultural beliefs through education would help increase public acceptance of oral rehydration and early refeeding to treat acute gastroenteritis. Primary care providers are in a unique position to help educate families about the value of these therapies. Incorporating a few remarks about diarrhea treatment as anticipatory guidance during a well-child visit would help dispel some common myths. Printed brochures are another potentially helpful method of patient education. Community health lectures by pediatricians and media interviews are powerful methods to spread the word about successful diarrhea treatment.
Improvements in ORS. Advances in both the composition of ORS and the pharmacotherapy of diarrhea should help address lingering concerns about shortening the duration of diarrhea episodes. Reducing the osmolarity of ORS would not only provide adequate rehydration, but would also decrease the volume of loose stools. A recent trial of such a solution noted a decrease in the use of IV therapy and no change in the incidence of hyponatremia.12 The addition of food-based polymers from rice or soy to ORS has also shown promise, as have small, single amino acids.
Probiotic therapy (the use of living microorganisms that, when digested in certain numbers, exert health benefits beyond inherent basic nutrition) has been studied as an adjunct to ORS. Lactobacillus has been shown to enhance mRNA expression of mucin, alter microflora, and compete with toxic bacteria for binding sites. Lactobacillus seems to be effective in acute and chronic gastroenteritis but not bacterial gastroenteritis. Many strains of Lactobacillus exist, but strains GG and LB are heat resistant and known to colonize the GI tract. Government regulation of probiotic therapy is not nearly as rigorous as regulation of the pharmaceutical industry. Patients should be counseled about reputable sources of Lactobacillus and warned to be cautious about outrageous health claims attributed to probiotic therapy. Lactobacillus GG, produced by Culturelle, has been studied in children with diarrhea.
Fiber supplementation also may help to shorten the duration of diarrhea disease. Guar gum added to ORS or pectin added to the diet with refeeding have both shown promise. A "super ORS" of the future might contain plant polysaccharides, glycine, Lactobacillus, guar gum, and pectin. Such an ORS would produce more formed stools and shorten the duration of diarrhea. In developing countries, innovations in the composition of ORS are likely to have the disadvantage of introducing new barriers of cost and availability. In developed countries, however, the addition of "high-tech" additives and higher cost will likely move ORS closer to the pharmacotherapy that many parents expect from modern health care. Paradoxically, more parents might accept the use of ORS as a result.
New medications for treating diarrhea, though not yet approved for use in children, might prove safer and more effective than those currently available. Racecadotril is an enkephalinase inhibitor that acts as a specific antisecretory agent in the GI tract. It has been shown to reduce stool output and the number of days to the first formed stool.13,14 Smectite is a mineral adsorbent that reduces luminal levels of toxins and inflammatory mediators. A trial of smectite in Italian children demonstrated a significant reduction in stool frequency and time to first formed stool.15 Loperamide-N-oxide is the prodrug of loperamide. It may be effective at lower doses and have fewer adverse effects than the parent drug. Zaldaride maleate is an intestinal calmodulin inhibitor that has shown promise in the treatment of diarrhea.
Not quite there, but on the way
Oral rehydration and early refeeding have significantly advanced the treatment of acute gastroenteritis in children. Implementing routine use of these therapies has proved to be a challenge, however. Improvements in the composition of ORS combined with vigorous patient education should further advance the treatment of this common, high-morbidity disease.
REFERENCES
1. Claeson M, Merson MH: Global progress in the control of diarrheal diseases. Pediatr Infect Dis J 1990; 9(5):345
2. Glass RI, Lew JF, Gangarosa RE: Estimates of morbidity and mortality rates for diarrheal disease in American children. J Pediatr 1991;118(4):S27
3. Parashar UD, Holman RC, Clark MJ, et al: Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: Surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis 1998;177:13
4. Parashar UD, Chung MA, Holman RC, et al: Use of state hospital discharge data to assess the morbidity from rotavirus diarrhea to monitor the impact of a rotavirus immunization program: A pilot study in Connecticut. Pediatrics 1999;104:489
5. UNICEF: The State of the World's Children 1998. New York, Oxford University Press, 1997
6. Zimmerman CM, Bresee JS, Parashar UD, et al: Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J 2001;20(1):14
7. Centers for Disease Control and Prevention: Preliminary FoodNet data on the incidence of foodborne illnessesSelected sites, United States 2002. MMWR Morb Mortal Wkly Rep 2003;52(15):340
8. American Academy of Pediatrics, Committee on Infectious Diseases: 2000 Red Book: Report of the Committee on Infectious Diseases, ed 25. Elk Grove Village, Ill., American Academy of Pediatrics, 2000
9. MacKenzie WR, Hoxie NJ, Proctor ME, et al: A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med 1994;331:161
10. Snyder JD: Use and misuse of oral therapy for diarrhea: Comparison of US practices with American Academy of Pediatrics recommendations. Pediatrics 1991;87(1):28
11. Szajewska H, Hoekstra JH, Sandhu B: Management of acute gastroenteritis in Europe and the impact of the new recommendations: A multicenter study. J Pediatr Gastroenterol Nutr 2000;30:522
12. CHOICE Study Group: Multicenter, randomized, double- blind clinical trial to evaluate the efficacy and safety of a reduced osmolarity oral rehydration salts solution in children with acute watery diarrhea. Pediatrics 2001; 107:613
13. Cezard JP, Duhamel JF, Meyer M, et al: Efficacy and tolerability of racecadotril in acute diarrhea in children. Gastroenterology 2001;120(4):799
14. Salazar-Lindo E, Santisteban-Ponce J, Chea-Woo E, et al: Racecadotril in the treatment of acute watery diarrhea in children. N Engl J Med 2000;343(7):463
15. Guarino A, Bisceglia M, Castellucci G, et al: Smectite in the treatment of acute diarrhea: A nationwide randomized controlled study of the Italian Society of Pediatric Gastroenterology and Hepatology (SIGEP) in collaboration with primary care pediatricians. SIGEP Study Group for Smectite in Acute Diarrhea. J Pediatr Gastroenterol Nutr 2001;32(1):71
SUGGESTED READING
AAP Subcommittee on Acute Gastroenteritis: Practice parameter: The management of acute gastroenteritis in young children. Pediatrics 1996;97:424
Ladinsky M: The cost effectiveness of oral rehydration therapy for US children with acute diarrhea. Medical Interface 1996;9:113
Northrup RS, Flanigan TP: Gastroenteritis. Pediatr Rev 1994;15:461
Reis EC, Goepp JG, Katz S, et al: Barriers to use of oral rehydration therapy. Pediatrics 1994;93:708
Santosham M, Fayad I, Abu Zikri M, et al: A double-blind clinical trial comparing World Health Organization oral rehydration solution with a reduced osmolarity oral rehydration solution containing equal amounts of sodium and glucose. J Pediatr 1996;128:45
DR. BERMAN is chief, division of pediatric gastroenterology, Ronald McDonald Children's Hospital at Loyola University Medical Center, and attending gastroenterologist, Advocate Lutheran General Children's Hospital, Park Ridge, Ill. He has nothing to disclose in regard to affiliations with, or financial interests in, any organization that may have an interest in any part of this article.
GUIDE FOR PARENTS
When your child has diarrhea
Diarrhea (loose, watery bowel movements) is a common problem in young children. It may be caused by a serious illness, but it usually results from a minor infection.
The danger of having diarrhea is becoming dehydrated (dried out). Your child may become very sick if he or she loses too much fluid and becomes dehydrated. Dehydration can usually be prevented by increasing the amount of liquid your child drinks. You may need to cut down on solid foods for 24 to 48 hours so that your child drinks more liquids.
We have examined your child and feel that the diarrhea can be treated at home. The following suggestions may be helpful to you in caring for your child.
What to feed your child




When to call the doctor
Call your child's doctor if any of the following occur:
Your child suddenly develops a high fever (101º F) taken under the arm.
Stomach pain becomes severe or is more than occasional cramps.
The diarrhea become bloody (more than a streak of blood).
The diarrhea becomes more frequent or more severe.
The child becomes dehydrated (see signs of dehydration below).
No improvement occurs within 24 to 48 hours.
You have any other concerns or questions.
Signs of dehydration
The soft spot on top of your infant's head (fontanelle) is sunken.
Your child has not urinated (passed water) for six hours.
Your child sheds no tears when he cries.
Your child's mouth is dry or sticky to the touch.
Your child's eyes are sunken, and the skin around the eyes is dark.
Your child is less active than usual or is difficult to wake up.
Preventing infection of other family members
When diarrhea is caused by a germ that is contagious, you can help to protect other family members by following these suggestions:
Keep your child away from other family members as much as possible.
Use separate eating utensils for your child and wash them with hot soapy water before they are used by others.
Wash your hands after touching your child, his eating utensils, or his soiled laundry.
Wash your child's soiled laundry separately in hot water.
Keep the toys that your child plays with separate and wash them with soap and water when possible.
Use separate washcloths to clean your baby after diaper changes.
Wash your hands well after each diaper change.
Clean the toilet often.
Your child may eat and drink his or her usual foods.
Your child should eat or drink only the foods checked below.
CAUTION!
Do not use stool "binders" or antidiarrhea medicines for children under 6 years of age unless your child's doctor specifically directs you to do so. These medicines can be very dangerous if they are not used properly.
Adapted with permission from Diarrhea. Homegoing Education and Literature Program, Children's Hospital, Columbus, Ohio, 1993
This guide may be photocopied and distributed without permission to give to your patients and their parents. Reproduction for any other purpose requires express permission of the publisher.