Infant with Hypotonia, Hypogonadism, and Developmental Delay
Low muscle tone, delayed motor milestones, and failure to gain weight. The mother was 38 at the time of delivery, but nothing else is noteworthy about this 9-month-old.

You have been following a 9-month-old with low muscle tone, delayed motor milestones, and failure to gain weight. Her family and gestational histories are normal except that her mother was 38 at the time of pregnancy; the child has 4 healthy siblings. The delivery was complicated by long labor and resuscitation was required, with NICU treatment for meconium aspiration. The newborn had significant hypotonia that necessitate tube feeding for a few days, but she was able to bottle-feed by the time of NICU discharge at age 7 days.
Current measurements show a length at the 10th centile for age, a weight just below the 3rd centile, and a head circumference also below the 3rd centile. You have watched her slow weight gain without prompting evaluation because it has paralleled the growth curve, and you have followed sluggish motor milestones with rolling back to front at 4 months and sitting at 8 months. She has appeared alert with good interaction and babbling.
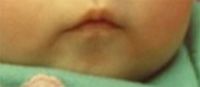
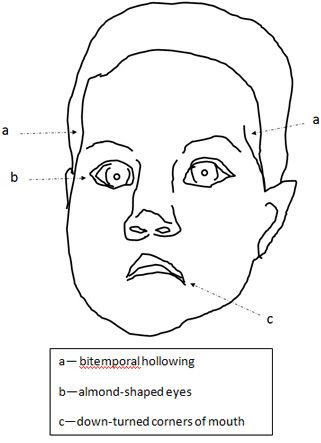
The pictures show a slightly unusual facial appearance, with almond-shaped eyes, down-turned corners of the mouth, and bitemporal hollowing. She also has somewhat unusual genitalia with a question of hypoplastic labia that make the clitoris easily visible.
Should you:
A. Initiate referral at this time, and to which subspecialty?
B. Suspect any particular diagnosis?
C. If there is a likely diagnosis, what diagnostic test would confirm it?
(Please click here for answers and discussion.)
ANSWERS:
A. Referral for developmental concerns is always a judgment call, but the subtle dysmorphology in this case might prompt referral to pediatric genetics rather than neurology or developmental pediatrics.
B. Although hypotonia has a huge range of central and peripheral neuromuscular causes, the early hypotonia, facial changes, and hypogonadism suggest the diagnosis of Prader-Willi syndrome. The “advanced” maternal age is likely coincidental although it can be associated with trisomy 15 conception with loss of father’s 15 chromosome to cause uniparental disomy.
C. About 60% of children with Prader-Willi syndrome have a microdeletion of chromosome 15 on the homologue that was inherited from father. The remainder have other deficiencies of paternal imprinting, either inheriting 2 copies of mother’s 15 (uniparental disomy) or a paternal chromosome 15 that did not acquire the male imprint (abnormal DNA methylation). The Prader-Willi DNA methylation test will detect all 3 causes of Prader-WIlli or its complement-the failure of female chromosome 15 imprinting known as Angelman syndrome.
Clinical features and diagnosis
Prader-Willi syndrome, first reported in 1956, is also referred to as Prader-Labhart-Willi syndrome or asthe hypotonia-hypomentia-hypogenitalism-obesity (HHHO) syndrome.1 It has an incidence of 1 in 16,000 to 25,000 births, usually sporadic (isolated cases).1,2 Approximately 70% of patients have microdeletion of chromosome 15, while the remaining 30% have either uniparental disomy for the maternally derived chromosome 15 or a failure of genomic imprinting. The latter is a mechanism for gene regulation that ensures complementary expression of genes on the maternal and paternal homologues of each chromosome pair.
Deletion may be demonstrated by targeted fluorescent in situ hybridization (FISH) testing showing a missing signal on one chromosome 15. Alternatively, one may order routine chromosomes with reflex to (proceed if prior test normal) array-comparative genomic hybridization (aCGH-also known as microarray analysis or CMA) to demonstrate any subtle deletion or duplication (eg, those for DiGeorge, Williams, Prader-Willi, etc). The Prader-WIlli/Angelman DNA methylation test will detect altered methyl patterns in all 3 types of Prader-Willi (or Angelman) syndrome, whether the altered pattern is due to deletion on the paternal 15 homologue, two copies of mother’s 15 homologue (uniparental disomy), or failure of father’s 15 homologue to be properly imprinted. Parental recurrence risks are usually very low (less than 1 in 1000) and parental studies are not required unless rare translocation of the 15q11 region is found.1-3
Clinical diagnosis of Prader-Willi syndrome should be suspected in neonates with hypotonia and hypogonadism, although the latter may be subtle in females.1 The facial changes reflect the degree of hypotonia, with bitemporal hollowing and down-turned corners of the mouth. The almond-shaped eyes may help with recognition, but the diagnosis is missed in many infants until the remarkable hyperphagia manifests between 1 and 6 years.
Early differential diagnosis includes myopathies that may be distinguished by abnormal CPK levels and nerve-muscle studies. Later differentials may include other conditions with obesity and developmental delay (Bardet-Biedl with polydactyly, Cohen with prominent incisors and eye changes, Simpson-Golabi-Behmel with its “bulldog” facies). However, the remarkable hyperphagia of Prader-Willi syndrome is unique.
Consensus diagnostic criteria include neonatal/infantile hypotonia with its associated facial features, infantile feeding problems, excessive weight gain between ages 1 to 6 years, hypogonadism, global developmental delay/mental disability, and hyperphagia/food obsession.1,2 Other findings include short stature with small hands and feet, pale hair and complexion (in the deletion form), obstructive or central sleep apnea, strabismus; thick and stringy saliva; recurrent picking at the skin to cause sores and infections, speech articulation problems, and stubborn behaviors with affinity for working puzzles.
Preventive health care
Attention to infantile “failure to thrive” is important since the weak suck and thick saliva pose challenges for feeding. The most critical issue is family counseling once the hyperphagia appears because unrestrained eating will lead to early death from morbid obesity. The voracious appetite reflects altered hypothalamic response to satiety, and these patients never feel full. Abnormal eating behaviors include stealing food, nocturnal foraging for food, eating inappropriate foods, and binge eating. The challenge can be summarized as “cherchez la grandmere,” since well-intentioned relatives often cannot resist a hungry child and undermine parental restraints. Nutritional counseling of the entire family is needed with dramatization of calories as death-a team approach involving nutritionists and behavioral modification strategies is often needed, ranging from rewards for weight maintenance to strategies like locking refrigerators or restricting movements at night. Even with the best of counseling, some parents decide to take chances with morbid obesity rather than constantly disappointing a moderately impaired and always hungry child.
Other complications include strabismus that may relate to the oculocutaneous albinism that occurs in deletion patients (a pink gene causing hypopigmentation resides in the Prader-Willi deletion region). Increased dental caries and enamel hypoplasia may reflect frequent high-carbohydrate diets, thick saliva , and rumination (10% of 17% of patients) in Prader-Willi syndrome.1-3 Anesthesia includes risks of aspiration pneumonitis, cor pulmonale, temperature instability, and cardiac arrhythmias.
Hypogonadotrophic hypogonadism with irregular menses and infertility joins hyperphagia, disruption of the sleep cycle, and temperature instability as evidence of hypothalamic dysfunction. Diabetes mellitus (5% to 15%) accompanies cardiopulmonary and sleep disturbances secondary to obesity.
Behavior problems include violent outbursts, temper tantrums, obsessive-compulsive behavior, rigidity, manipulation, and stubbornness in young children. Autistic behaviors occur in 19% of children with deletion and 38% of those with uniparental disomy. Older children and adults exhibit depression and a “refusal-lethargy syndrome” of hyperkinesis, manipulative refusal of food and drink, and deliberate soiling. These may be exacerbated by daytime fatigue due to obstructive or central sleep apnea. Developmental disabilities are usually moderate: the ability to sit occurs at an average age of 12 to 13 months, walking at 24 to 30 months, and riding a tricycle at 4.2 years.1 Cognitive functions include single words appearing at 21 to 23 months and sentences at a mean of 3.6 years. Articulation defects with nasal speech occur. Reading is a relative strength; mathematics and social interactions are weaknesses. A global IQ above 70 occurs in 40% of patients.1-3
After diagnosis, a referral to ophthalmology and endocrinology/nutrition can optimize vision. Counsel for weight control and consider growth hormone therapy (as early as age 2) to enhance stature and muscle strength.3 Developmental-behavioral pediatricians can help coordinate early childhood intervention and preschool therapies, assisting with autism assessment, eating behavior modification, and medication management.
The parent association and its website provide an important resource for Prader-Willi families and their physicians.4
References:
References
1. Holm VA, Cassidy SB, Butler MG et al. Prader-Willi syndrome: Consensus diagnostic criteria. Pediatrics. 1993;91:398-402.
2. Wilson GN, Cooley WC. Preventive Health Care for Children with Genetic Condition: Providing a Medical Home. 2nd ed. Cambridge: Cambridge University Press, 2006:241-246.
3. Prader-Willi Syndrome Association USA. www.pwsausa.org/syndrome/index.htm.
4. Veltman MW, Craig EE, Bolton PF. Autism spectrum disorders in Prader-Willi and Angelman syndromes: a systematic review. Psychiatr Genet. 2005;15:243-254.