Is it the flu?
When and how to use rapid testing for influenza
In late 2017, the US Centers for Disease Control and Prevention (CDC) released a health advisory related to increased activity of influenza A (H3N2) viruses. In previous years, the H3N2 strain has been associated with decreased vaccine effectiveness and increased rates of hospitalization and deaths among pediatric patients.1 It is important for the pediatrician to understand the strengths, weaknesses, and limitations of current testing for influenza as well as identify testing modalities that might be more appropriate for their clinics in the future.
Recommendations for treatment of influenza
Given current recommendations for treatment of influenza in children,2 the pediatrician needs to consider the operating characteristics and limitations of influenza testing in making clinical decisions to test and treat. Because treatment should be offered or considered for any of the groups in Table 1, testing also needs to be considered for these groups.
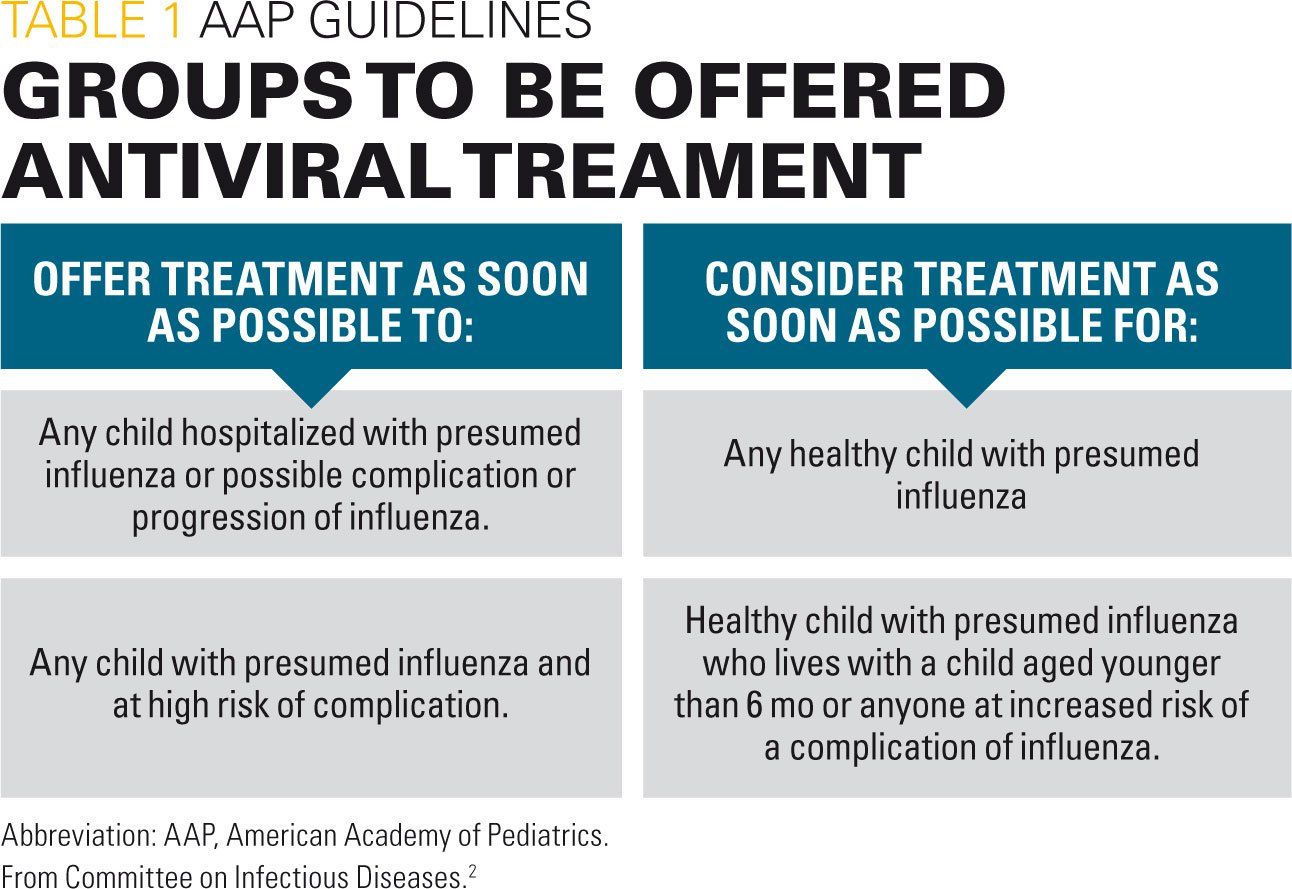
Underidentification and overtreatment
Although influenza testing and treatment is recommended for a number of different groups, clinical practice appears to be suboptimal.3,4 Among children evaluated at 7 hospitals of the New Vaccine Surveillance Network during the 2015-2016 flu season, 52% of children (range 25%-84%) admitted for influenza were treated with antivirals.5
Recommended: Using a patient's genetics to guide prevention, diagnosis, and treatment
Placing an increased emphasis on testing can result in:6
· Rapid diagnosis and treatment with antiviral medication.
· Fewer additional diagnostic tests.
· Fewer hospitalizations.
· More appropriate infection control for hospitalized patients.
· More appropriate antibiotic use.
Recommendations for influenza testing
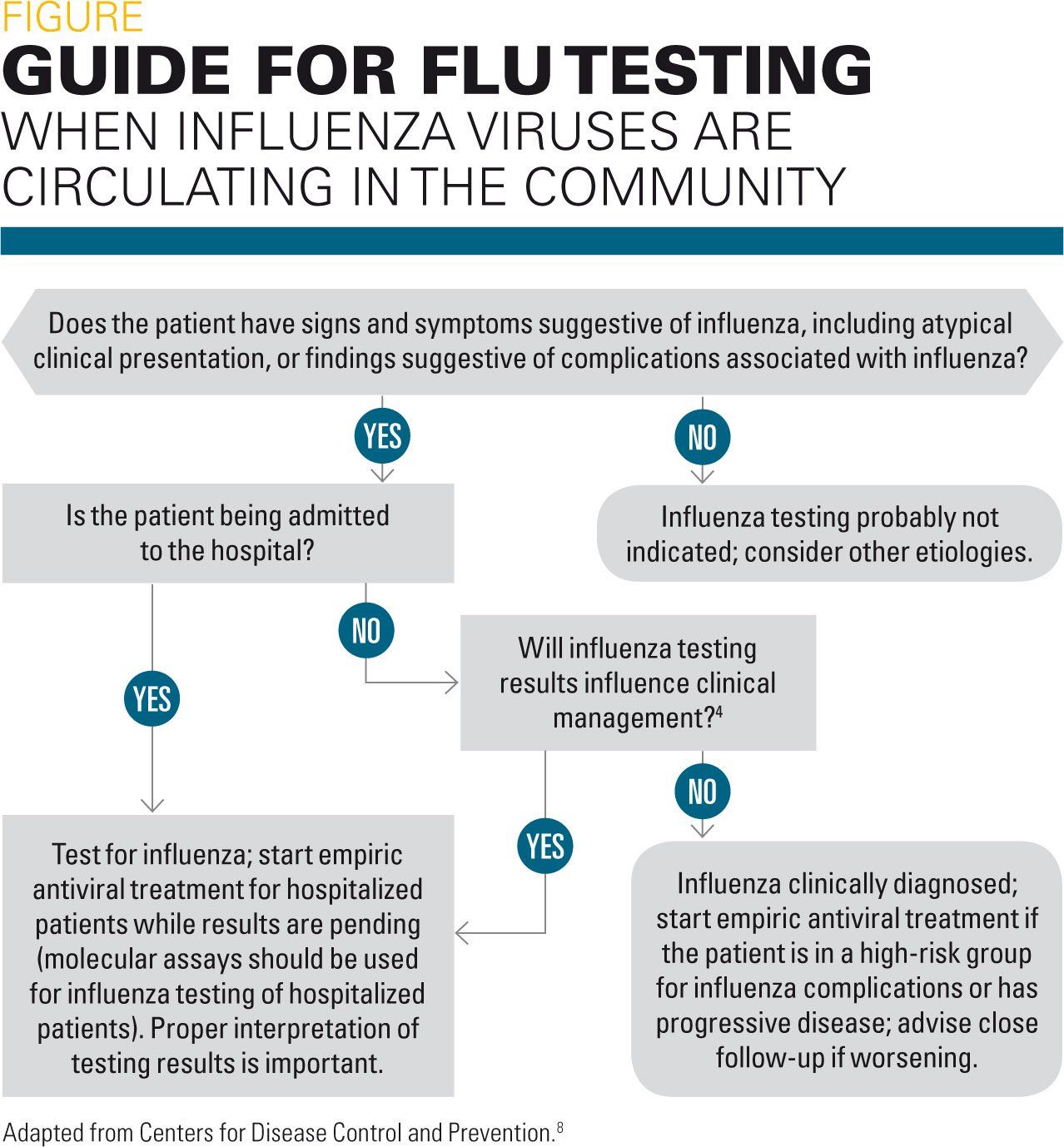
Laboratory testing for influenza virus should be undertaken when a child has flu symptoms and the results will impact management of the patient, including those at high risk.2,7 See Table 2 for children to consider for testing. The CDC recommendations for testing are depicted in the Figure.8
Some key points to remember include:2,7,9
· Laboratory confirmation of influenza is not needed to initiate treatment.
· Antiviral prescriptions do not need to be delayed until confirmation of influenza by diagnostic testing, especially if flu is in the community and the patient has signs and symptoms compatible with the flu.
· Patients at high risk of significant morbidity or complications should be treated based on clinical suspicion and influenza activity in the community.
· Vaccination does not exclude a diagnosis of influenza.

NEXT: Available testing methods
Available testing methods
Depending on the testing modality, an influenza diagnosis depends on the identification of virus or detection of viral protein or viral RNA in respiratory tract secretions.6 Negative antigen tests do not rule out an influenza infection.
The pediatrician and office staff need to be cognizant of appropriate specimen collection and its impact on influenza diagnosis. An inadequate specimen (right site but too few cells), inappropriate specimen (generally nasopharyngeal specimens are preferred), incorrect transport medium or dilution, and improper storage or transport of specimens all can lead to an inaccurate diagnostic test.10
RT-PCR assays
Reverse transcriptase polymerase chain reaction (RT-PCR) assays are molecular assays that have good sensitivity (86%-100%)2and make a diagnosis by identifying influenza viral RNA in respiratory specimens. Currently, RT-PCR is considered the gold standard test, but these samples are usually required to be sent to specialized laboratories, and processing can take 1 to 8 hours. Results are not generally available at the conclusion of an outpatient encounter.
Consequently, rapid influenza diagnostic tests (RIDTs) utilizing viral antigen detection are much more commonly performed in the outpatient setting because results are provided more quickly and before the end of the visit. Additionally, RT-PCR testing is generally more expensive than other testing methods.6,11
False-positive and false-negative RT-PCR assays are relatively rare. Other advantages of RT-PCR tests include the ability to detect influenza viral RNA for longer periods of time compared with other tests. However, a positive RT-PCR test does not necessarily indicate viable virus or ongoing influenza viral replication. Some RT-PCR assays can detect influenza A or B viruses, influenza A subtypes, and novel influenza A virus infections.11
Rapid molecular assays
Two rapid molecular assays (Alere Influenza A & B Test [Abbott; Abbott Park, Illinois] and cobas Liat PCR System [Roche Diagnostics; Indianapolis, Indiana]) are Clinical Laboratory Improvement Amendments (CLIA)-waived and can be used at point of care in a pediatrician’s office or emergency department (ED). Sensitivity is slightly lower than RT-PCR (66%-100%),11 but a recent meta-analysis found sensitivity of 92% and 87% for influenza and B, respectively.6 Sensitivities also were generally higher in children compared with adults as well.6,11 The main advantage of these assays over RT-PCR is the ability to perform testing at the point of care, and results are available within 20 minutes.
More: 3 steps to boost health literacy
It is important to obtain a patient’s history of vaccination because live attenuated vaccine virus strains (not recommended for the 2017-2018 influenza season) may give a positive PCR test from 3 to 10 days after administration.
RIDTs
Rapid influenza diagnostic tests are immunoassays that detect influenza A and B viral nucleoprotein antigens in respiratory specimens. Many of these tests are CLIA-waived and can yield a result in less than 15 minutes, but they also have a lower sensitivity (50%-70%).6,12 However, in studies in which only children are tested, the sensitivity appears to be at the higher end of reported sensitivity. It is thought that higher viral loads and increased viral shedding in pediatric patients account for this.13
The risk of a false-negative test is significantly greater with this test and may occur even when incidence of influenza in the community is high. If signs, symptoms, and clinical examination all point to influenza, the pediatrician should not be dissuaded by a negative RIDT.6,12
There is also a risk of false-positives, especially when incidence of influenza is low in the community. As a result, the pediatrician cannot exclude influenza with a negative test, only confirm with a positive test. In addition, RIDT testing is limited by onset of symptoms, with false-negatives more likely to occur as time increases from symptom onset to testing.6,12
NEXT: More testing choices
Direct and indirect IF assays
Immunofluorescence (IF) studies rely on the use of antibodies to apply fluorescent dye to a target antigen. Cells are affixed to glass slides and stained with antibodies. Slides are then reviewed under a fluorescence microscope to identify viral protein in infected cells. In direct IF assays, a single antibody is used against the antigen of interest whereas indirect IF assay utilizes multiple antibodies. Additional testing is then needed to identify the influenza strain. These tests require significant technical expertise and 1 to 2 hours of processing time.10 Immunofluorescence studies have a sensitivity in the 70% to 100% range, good specificity, and they perform better than RIDT studies.2,7,12
DIAs
Digital immunoassays (DIAs), also known as rapid immunochromatographic antigen detection assays, improve upon IF technology using digital analysis by performing an automated scan of a test strip and processing the result. Digital immunoassays eliminate the need for a lab technician to review and interpret the result.14
Two DIAs have been commercialized and granted CLIA waivers: BD Veritor (BD Diagnostics; Sparks Glencoe, Maryland) and Sofia (Quidel Corporation; San Diego, California). Similar to rapid molecular assays, these DIAs are able to be used at the point of care in a pediatrician’s office or ED. These tests produce results in less than 15 minutes but are slightly less sensitive than the rapid molecular assays (80% and 77% for influenza A and B, respectively). As with the rapid molecular assays, sensitivity appears to be higher in children compared with adults.6,11
Viral culture
The RT-PCR assay supplanted viral culture as the gold standard test for influenza, but viral culture is still commonly used as a comparator in studies of new commercial tests. Because results are not available for 10 to 14 days, clinical utility for influenza is limited. Although rapid culture assays are now available, results are still not available for 24 to 48 hours. One potential clinical use of viral culture is the confirmation of a negative RIDT.10
Serologic testing
Serologic testing looks for a minimum fourfold rise in antibody titers when comparing acute and convalescent samples to make a diagnosis of influenza. As such, it is not helpful in the clinical decision-making process related to influenza acutely. However, if making a diagnosis serves a particular clinical purpose, serologic testing may be of benefit.
Interpretation of test results
If the influenza test is negative, the pediatrician cannot rule out an influenza infection if the test has a low sensitivity or if the specimen was collected a significant amount of time after onset of symptoms. Signs, symptoms, and risk of complications should be considered to determine if antiviral treatment needs to be initiated.15
If the influenza test is positive, the pediatrician should begin antiviral treatment as soon as possible. Of note, dual infections with influenza A and B rarely occur together. If testing is positive for both strains, the specimen should be referred to the public health department for testing. Additionally, referral to the health department may be needed if the clinical situation requires subtyping of influenza A virus, differentiation between influenza A and B, or other analyses are needed.15
If there is a concern for coexisting bacterial infection, the pediatrician needs to consider coverage for Streptococcus pneumoniae, Staphylococcus aureus (including MRSA), and group A Streptococcus.15
Next: How to help parents cut healthcare costs
If the influenza test is negative, the pediatrician cannot rule out an influenza infection, especially if the pediatrician is utilizing RIDT or another test with low sensitivity. Similarly, if the patient is presenting 4 or more days after symptom onset, the negative test could be a false negative. The pediatrician should use clinical signs and symptoms along with local influenza activity to decide if antiviral treatment is indicated. If the patient is being admitted to the hospital, it is advisable to begin therapy without waiting for testing results if the pediatrician suspects influenza. Similarly, the high-risk patient or any patient with progressive disease also is a candidate for empiric treatment.15

References
1. Centers for Disease Control and Prevention. 12/27/2017: Lab advisory: Seasonal influenza A (H3N2) activity and antiviral treatment of patients with influenza. Available at: https://www.cdc.gov/ophss/csels/dls/locs/2017/H3N2-antiviral-treatment.html. Updated January 29, 2018. Accessed February 5, 2018.
2. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2017-2018. Pediatrics. 2017;140(4):e20172550.
3. Ison MG. Contemporary influenza diagnostics: renewed focus on testing patients. Ann Intern Med. 2017;167(6):438-439.
4. Ison MG. Editorial commentary: failing our patients by suboptimally treating influenza infections. Clin Infect Dis. 2014;59(6):783-786.
5. Campbell AP, McGowan C, Rha B, et al. Influenza clinical diagnostic testing and antiviral treatment among children hospitalized with acute respiratory illness during the 2015-16 influenza season. Open Forum Infect Dis. 2017;4(suppl 1):S356.
6. Merckx J, Wali R, Schiller I, et al. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167(6):394-409.
7. Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children-diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003-1032.
8. Centers for Disease Control and Prevention. Influenza (Flu). Guide for considering influenza testing when influenza viruses are circulating in the community. Available at: https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm. Updated November 2, 2016. Accessed February 5, 2018.
9. Baron EJ, Miller JM, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57(4):e22-e121.
10. Peaper DR, Landry ML. Rapid diagnosis of influenza: state of the art. Clin Lab Med. 2014;34(2):365-385.
11. Centers for Disease Control and Prevention. Guidance for clinicians on the use of RT-PCR and other molecular assays for diagnosis of influenza virus infection. Available at: https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm. Updated October 25, 2016. Accessed February 5, 2018.
12. Centers for Disease Control and Prevention. Guidance for clinicians on the use of rapid influenza diagnostic tests. Available at: https://www.cdc.gov/flu/pdf/professionals/diagnosis/clinician_guidance_ridt.pdf. Accessed February 5, 2018.
13. Kondrich J, Rosenthal M. Influenza in children. Curr Opin Pediatr. 2017;29(3):297-302..
14. Dunn JJ, Ginocchio CC. Can newly developed, rapid immunochromatographic antigen detection tests be reliably used for the laboratory diagnosis of influenza virus infections? J Clin Microbiol. 2015;53(6):1790-1796.
15. Centers for Disease Control and Prevention. Algorithm to assist in the interpretation of influenza testing results and clinical decision making during periods when influenza viruses are circulating in the community. https://www.cdc.gov/flu/professionals/diagnosis/algorithm-results-circulating.htm. Updated November 2, 2016. Accessed February 5, 2018.
Dr Bass is chief medical information officer and professor of Medicine and of Pediatrics, Louisiana State University Health Sciences Center–Shreveport. He has nothing to disclose in regard to affiliations with or financial interests in any organizations that may have an interest in any part of this article.