J&J submits applications for guselkumab to treat plaque psoriasis, jPsA in children
The submission to treat PsO is for children aged 6 years and up while the jPsA submission is to treat children aged 5 years and older.
J&J submits applications for guselkumab to treat plaque psoriasis, jPsA in childrenLatest revision | Image Credit: © pimentos - © pimentos - stock.adobe.com.
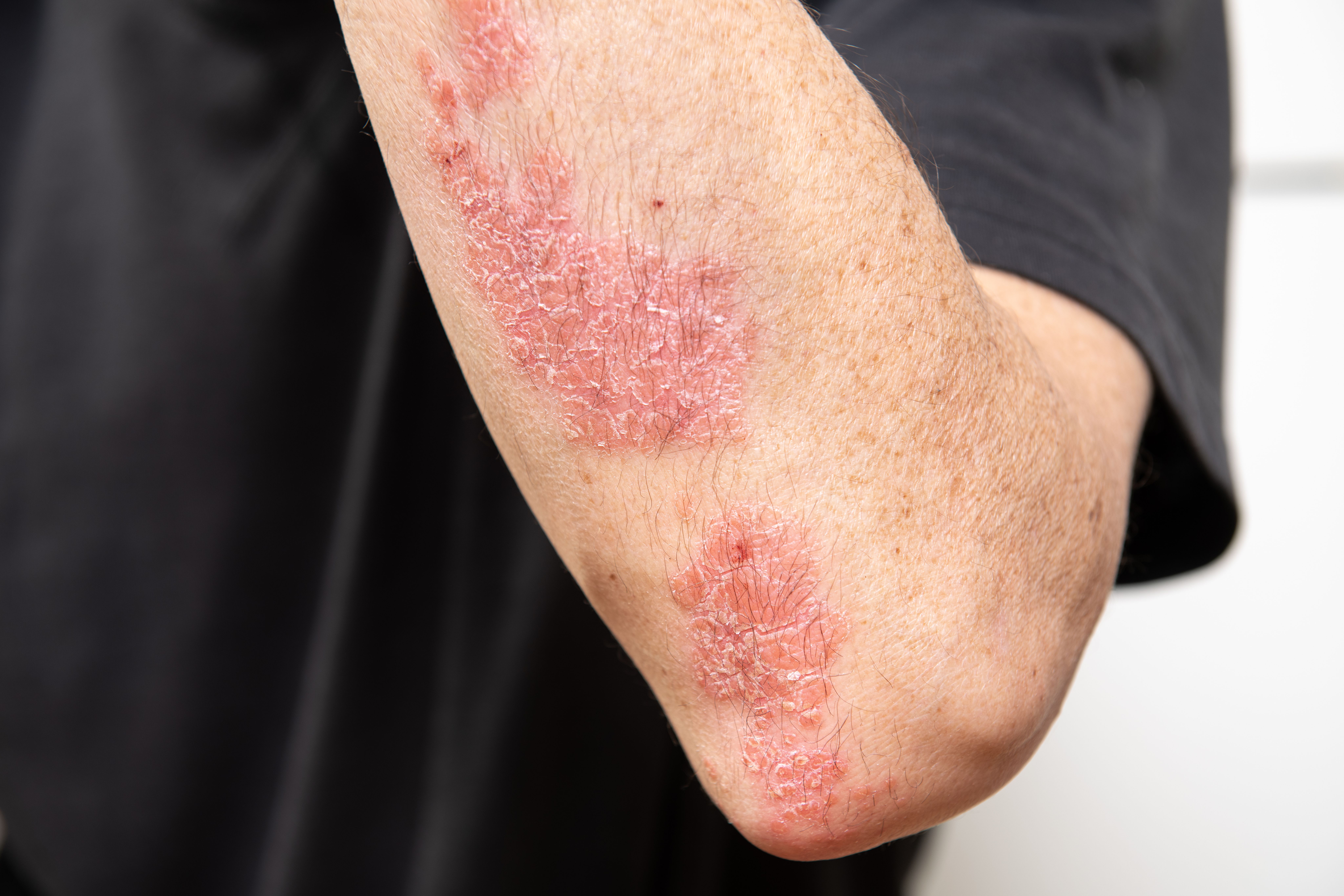
A pair of supplemental Biologics License Applications (sBLAs) have been submitted to the FDA for Johnson & Johnson's guselkumab (Tremfya) to treat moderate-to-severe plaque psoriasis (PsO) in children aged 6 years and up and to treat active juvenile psoriatic arthritis (jPsA) in children aged 5 years and older, Johnson & Johnson recently announced.1
According to the announcement, the PsO submission is backed by data from the phase 3 PROTOSTAR study (NCT03451851) that featured pediatric patients with moderate-to-severe plaque PsO. In addition, the submission is based on bridging pharmacokinetic (PK) data from the phase 3 Voyage 1 (NCT02207231) and Voyage 2 (NCT02207244) studies in adult patients.1
For the jPsA indication, the submission was based on the PK extrapolation analyses from the adult PsA studies, Discover 1 (NCT03162796) and Discover 2 (NCT03158285), while safety and efficacy data was from the Protostar study, according to Johnson & Johnson.1
"This milestone underscores our commitment to transform the standard of care for patients of all ages and builds on our expertise and legacy in IL-23 and immune-mediated diseases,” Liza O’Dowd, MD, vice president, Immunodermatology Disease Area Leader, Johnson & Johnson Innovative Medicine, said in a statement.1
"There is a critical gap in the treatment of children and adolescents with these skin and joint conditions, where debilitating symptoms can present challenges related to physical appearance and ability to function. We are working to address this gap by investigating the efficacy and well-characterized safety profile of [guselkumab] for pediatric patients," said O'Dowd.1
In the phase 3 Protostar study, a randomized, multicenter, placebo- and active comparator-controlled study that evaluated efficacy, safety, and PK of subcutaneously-administered guselkumab to treat chronic PsO in pediatric patients, co-primary endpoints included the Investigator's Global Assessment 0/1, and Psoriasis Area and Severity Index 75 at week 16.1
In the phase 3 Discover-1 study, a multicenter, randomized, double-blind study that evaluated safety and efficacy of guselkumab administered as a subcutaneous injection in individuals with active PsA (including those previously treated with 1 or 2 tumor necrosis factor inhibitors), the primary endpoint was response of American College of Rheumatology 20 (ACR20) at week 24.1,3
ACR20, according to an article published in Arthritis Research & Therapy, is a multi-demensional outcome measure used to evaluate treatments in rheumatoid arthritis, one used throughout rheumatic diseases.3
jPsA, a form of juvenile idiopathic arthritis, is characterized by chronic joint inflammation and swelling, as well as an increased risk for asymptomatic eye inflammation, according to the Children's Hospital of Philadelphia. Signs and symptoms can rage from mild to severe, and can include swelling of the small and large joings, inflammation where tendons and ligaments attach to bones, morning stiffness, back pain or stiffness, and more.2
PsO, an immune-related disease, results in the overproduction of skin cells, which causes inflammed, scaly plaques that can be itchy or painful, stated Johnson & Johnson. "Almost one-third of PsO cases begin in childhood, with roughly 20,000 children under 10 diagnosed with psoriasis each year. Having visible skin disease can be highly stressful for children and adolescents and can have a long-term impact on those affected," the company wrote in the submission announcement.1
References:
1. Johnson & Johnson seeks US FDA approval for first pediatric indications for TREMFYA (guselkumab). Johnson & Johnson. Press release. December 2, 2024. Accessed December 3, 2024. https://www.jnj.com/media-center/press-releases/johnson-johnson-seeks-u-s-fda-approval-for-first-pediatric-indications-for-tremfya-guselkumab
2. Psoriatic arthritis in children. Children's Hospital of Philadelphia. Accessed December 3, 2024. https://www.chop.edu/conditions-diseases/psoriatic-arthritis-children
3. Felson DT, LaValley, MP. The ACR20 and defining a threshold for response in rheumatic diseases: too much of a good thing. Arthritis Res Ther. 2014 Jan 3;16(1):101. doi: 10.1186/ar4428. PMID: 24387346; PMCID: PMC3978644.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.















