At last: A vaccine for rotavirus
Rotavirus vaccine has just been added to the recommended immunization schedule. Learn the characteristics of this new vaccine and how to administer it in your practice.
At last: A vaccine for rotavirus
By Susan E. Coffin, MD, and Paul A. Offit, MD
Rotavirus vaccine has just been added to the recommendedimmunization schedule. Learn the characteristics of this new vaccine andhow to administer it in your practice.
Rotavirus gastroenteritis is the most common underlying cause of dehydrationamong young children in the United States. For most infected children, thedehydration is mild. Nevertheless, when severe dehydration requiring hospitalizationoccurs, rotavirus infection is the most frequent cause. The morbidity associatedwith the illness and the substantial direct and indirect costs it imposeshave impelled the search for a safe and effective vaccine. Last year, afterextensive clinical trials, a rotavirus vaccine was licensed by the Foodand Drug Administration (FDA). Called RotaShield, the new vaccine is includedin the routine immunization schedule for 1999 and has recently been recommendedfor routine use by the AAP's Committee on Infectious Diseases.We will examinethe case for immunizing infants against rotavirus infection, describe theproperties of the RotaShield vaccine, and provide guidance on how it isto be used.
Why immunization makes sense
The rationale for immunizing children against rotavirus infection hasbeen questioned. After all, rotavirus is not the only pathogen that causesdiarrhea in infants and young children. And even when rotavirus is the cause,most children in the US get over the infection with minimal distress. Furthermore,oral rehydration is an effective, simple treatment for diarrhea.
There are persuasive counters to that argument, however. First, dehydrationassociated with rotavirus can be particularly severe. Several features ofrotavirus disease contribute to its pathogenicity. Young children infectedwith rotavirus typically experience fever, vomiting, and diarrhea that lastthree to five days.1 As compared with other causes of infectiousenteritis, rotavirus has a unique propensity to cause vomiting. In fact,in approximately 50% of primary infections, vomiting may precede the onsetof diarrhea. In addition, infants and toddlers with rotavirus infectionmay have 10 or more loose, watery stools a day. Thus, fluid losses fromvomiting and diarrhea may be great and difficult to replace in young infantsand toddlers.
Second, rotaviruses are the most common causes of acute gastroenteritisin children. An estimated 3 million cases of rotavirus diarrhea occur inthe US each year. By 3 years of age, virtually all American children haveexperienced at least one rotavirus infection. The illness is seasonal; upto 90% of all rotavirus infections in the US occur during the winter months.From December to April, rotaviruses can be isolated from approximately 60%of stool specimens obtained from pediatric patients with gastroenteritis.
Children are most likely to come down with a clinically significant infectionbetween 6 and 24 months of age. Rotavirus infection does occur in the neonatalperiod, but it is often asymptomatic at that age. Immunity induced by naturalinfection is not long lasting, so that older children and adults may experiencerepeated infections.2 These secondary infections, however, aregenerally less severe, and the illness doesn't last as long. One of fiveparents caring for a child with rotavirus gastroenteritis will develop symptomaticdisease.
The medical and societal costs of rotavirus infections are tremendous.A 1995 study by the Viral Gastroenteritis Section of the Centers for DiseaseControl and Prevention found that annual US medical costs attributable torotavirus infections were greater than $550 million.3 Societalcosts, measured in wages lost by adult caretakers, were estimated to exceed$800 million.
Worldwide, rotaviruses are among the leading causes of childhood morbidityand mortality. Approximately 1 million children die each year of dehydrationdue to rotavirus infection. Many more suffer from acute and chronic malnutritiondue to repeated infections.
What a vaccine canand can'tdo
A realistic goal for a rotavirus vaccine would be to induce a level ofprotection comparable to that stimulated by natural infection. That protectionis not total. Pathogens like rotavirus that replicate only on the mucosalsurfaces of the gastrointestinal tract do not induce complete and lifelongimmunity.4 Studies of children who have had a natural rotavirusinfection show that approximately 80% are protected from moderate-to-severedisease for at least the next two years.2 A rotavirus vaccinemay be expected to induce a similar degree of protection against moderate-to-severerotavirus gastroenteritis, but not to prevent all cases of rotavirus diarrhea.However, if most moderate-to-severe rotavirus infections were prevented,the number of office visits and hospitalizations for gastroenteritis wouldbe markedly reduced.
The RotaShield vaccine
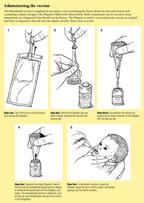
RotaShield is a live, attenuated oral rotavirus vaccine approved by theFDA for use in infants less than 1 year of age. It is lyophilized (freezedried) and supplied in single-dose vials together with diluent. The vaccineis reconstituted with 2.5 mL of citrate-bicarbonate solution, premeasuredand provided in an individual squeeze tube, the Dispette. This diluent buffersstomach acid so that pH-sensitive rotaviruses are not inactivated. The reconstitutedvaccine is withdrawn from the vial into the Dispette and administered orally;for a step-by-step explanation of how this is done, see the Box, "Administeringthe vaccine."
RotaShield, like oral polio vaccine (OPV), is a multivalent or compositevaccine. It contains four distinct rotavirus strains (G types 1, 2, 3, and4), named for important glyco-proteins found in the outer shell of the rotavirus.These proteins induce antibodies that neutralize viral infectivity. Theantigenic composition of the proteins of the predominant circulating strainof rotavirus varies according to location and season. Most human rotavirusdisease throughout the world is associated with five G types (1, 2, 3, 4,and 9). In the US, rotavirus serotypes G1, G2, G3, G4, and G9 predominate.
Three of the four viruses included in the vaccine are reassortant viruses,that is, viruses that combine proteins from two different types of rotavirus.These reassortant rotaviruses are primarily composed of a rotavirus thatinfects rhesus monkeys (RRV); RRV does not replicate efficiently in humansand thus does not usually cause diarrhea. However, vaccine viruses alsocontain a single protein derived from human rotaviruses; the gene segmentthat encodes for the human rotavirus G protein has been inserted to replacethe gene segment that encodes for simian rotavirus G protein. Because thesereassortant viruses are largely nonhuman, they are naturally attenuated;they do not cause rotavirus illness in humans. At the same time, they arehighly immunogenic because they contain a human rotavirus protein that stimulatesvirus neutralizing antibodies.
Three separate strains of reassortant rotaviruses have been made (see"How reassortant rotavirus strains are generated"). Each containsa different human G protein type (1, 2, or 4). The fourth component of RotaShieldis RRV alone. The simian G protein is similar to human G protein type 3and can induce antibodies that neutralize human rotavirus type G3. Thus,RotaShield is designed to induce protection from human rotaviruses typesG1, G2, G3, and G4.

RotaShield is given in three separate doses at six to 12-week intervals.5Multiple doses are necessary because some children do not mount an immuneresponse to all four vaccine components after the first or second dose.It is unclear why some children require three doses to respond to all fourvaccine components. Placentally derived maternal anti-rotavirus antibodiesmay interfere with the generation of rotavirus immunity. Alternatively,one strain of virus in the vaccine may interfere with the generation ofimmunity to another vaccine strain. Booster doses ensure that more than90% of completely immunized infants will have circulating antibodies capableof neutralizing all four of the major strains of rotavirus.
An immunologic correlate of protection from rotavirus infection has notbeen clearly defined.4 Antibodies present at the intestinal surfaceare likely to be an important component of rotavirus immunity, but at present,no data demonstrate intestinal antibody responses to RotaShield. However,50% to 70% of children had detectable serum rotavirus-specific IgA antibodiesafter receiving three doses, which suggests that the vaccine does stimulateintestinal IgA responses. In addition, rotavirus-specific serum neutralizingantibodies have been detected in up to 90% of vaccinated infants. However,when G-type specific neutralizing antibodies were measured, lower ratesof responses were observed.
Clinical trials in developed countries have consistently shown protectiveeffects of RotaShield. In placebo-controlled clinical trials with approximately1,500 American children, the protective efficacy against all rotavirus diseasewas 49% to 52%.6,7 When only severe or clinically significantrotavirus gastroenteritis was considered, efficacy was 70% to 80%. Theseresults indicate that while RotaShield will not prevent all episodes ofrotavirus-induced diarrhea, it will markedly reduce the frequency and durationof clinically important infection. When the vaccine comes into widespreaduse, many fewer infants and young children will require outpatient evaluationor hospitalization for dehydration.
How to use the vaccine
Immunization with RotaShield is recommended for infants at 2, 4, and6 months of age. The first dose of rotavirus vaccine can be given as earlyas 6 weeks of age or as late as 6 months. Initiation of the series after6 months of age is not recommended because it may result in a higher rateof fever. An interval of at least three weeks should elapse between doses.
Contraindications. Children who have demonstrated hypersensitivityreactions to neomycin, amphotericin B, or monosodium glutamate, which arecomponents of the vaccine, should not receive RotaShield. Nor should thevaccine be administered to children with known or suspected immunodeficiencies,as safety and efficacy in immunocompromised children have not been demonstrated.Short-term (less than two weeks), low-dose steroid therapy is not a contraindication.Infants with moderate or severe febrile illness should not receive the vaccineduring the acute phase of their illness, but should be immunized as soonas they have recovered.
Side effects. No long-term adverse effects have been observedin the several thousand infants who have participated in clinical trialsof the RotaShield vaccine over the past 10 years. However, some systemicside effects have been noted. Approximately 20% of infants receiving theirfirst dose of RotaShield develop a fever of more than 38° C, and 1%to 2% have fever higher than 39° C. In addition, some infants experienceincreased irritability and anorexia following immunization. If serious adverseevents occur, they should be reported to the Vaccine Adverse Events ReportingSystem (VAERS) by calling 800-822-7967, or via the World Wide Web at www.cdc.gov/nip/vaers.htm.
Because RotaShield is composed of infectious virus, some viral replicationdoes occur in the small intestine of vaccinated infants. At least 50% ofimmunized infants shed vaccine strains of rotavirus in their stools afterat least one of the three doses. At present the public health implicationsof fecal shedding of vaccine viruses are unclear. Fecal shedding may beadvantageous, inducing the same sort of contact immunity that has been ascribedto OPV. Household and day-care contacts of recently immunized infants maybecome infected with vaccine strains of rotavirus and thus develop immunity.The significance of unintentional transmission of vaccine viruses to immunocompromisedhousehold contacts is unknown. Additional information about administeringthe vaccine is provided in "Q and A on rotavirus vaccine.".

The future of rotavirus vaccines
Researchers at Wyeth-Lederle and elsewhere are continuing to work ondeveloping more effective and less reactive rotavirus vaccines. Ideally,a second-generation vaccine would prevent moderate-to-severe disease withoutthe first-dose side effects of fever, irritability, and anorexia inducedby the current vaccine. Current research strategies include bovine-humanrotavirus reassortant vaccine strains or attenuated human rotavirus as vaccinecandidates. At present, however, universal use of RotaShield will markedlyimprove the health of infants in this country.
THE AUTHORS:
DR. COFFIN is Physician, Division of Immunologic and InfectiousDiseases, The Children's Hospital of Philadelphia, and Assistant Professorof Pediatrics, University of Pennsylvania School of Medicine, Philadelphia.
DR. OFFIT is Chief, Section of Infectious Diseases, TheChildren's Hospital of Philadelphia, Associate Professor of Pediatrics,The University of Pennsylvania School of Medicine, and Adjunct AssociateProfessor, The Wistar Institute of Anatomy and Biology, Philadelphia.
REFERENCES
1. Ho K, Glass R, Pinsky P, et al: Rotavirus as a cause of diarrhealmorbidity and mortality in the US. J Infect Dis 1988;158:1112
2. Velazquez FR, Matson DO, Calva JJ, et al: Rotavirus infection in infantsas protection against subsequent infections. N EngI J Med 1996;335:1022
3. Smith JC, Haddix AC, Teutsch SK, et al: Cost-effectiveness analysisof a rotavirus immunization program for the US. Pediatrics 1995;96:609
4. Offit P: Host factors associated with protection against rotavirusdisease: The skies are clearing. J Infect Dis 1995;174(suppl 1):S59
5. Committee on Infectious Disease: Prevention of rotavirus disease:Guidelines for use of rotavirus vaccine. Pediatrics 1998;102:1483
6. Bernstein D, Glass R, Rodgers Q, et al: Evaluation of rhesus rotavirusmonovalent and tetravalent reassortant vaccines in US children. US RotavirusVaccine Efficacy Group. JAMA 1995;273:1191
7. Rennels M, Glass R, Dennehy P, et al: Safety and efficacy of high-doserhesus-human reassortant rotavirus vaccines: Report of the National MulticenterTrial. Pediatrics 1996;97:7
8. Tucker AW, Haddix AC, Bresee JS, et al: Cost-effectiveness analysisof a rotavirus immunization program for the US. JAMA 1998;279(17):1371
At last: A vaccine for rotavirus.
Contemporary Pediatrics
1999;0:105.