Article highlights
- Lebrikizumab Success: Lebrikizumab, combined with topical corticosteroids, effectively treated moderate-to-severe atopic dermatitis in adolescents and adults, even for those not responsive to cyclosporine.
- Study Highlights: Phase 3 ADvantage study showed significant skin clearance and itch relief in patients. Long-term responses were sustained in 84% of responders across various disease domains.
- EASI Scores: Lebrikizumab achieved mild or clear skin in over 50% of patients, demonstrating its effectiveness regardless of baseline severity.
- Development Ownership: Eli Lilly and Company has global rights, except in Europe where Almirall holds the rights for lebrikizumab's development and commercialization.
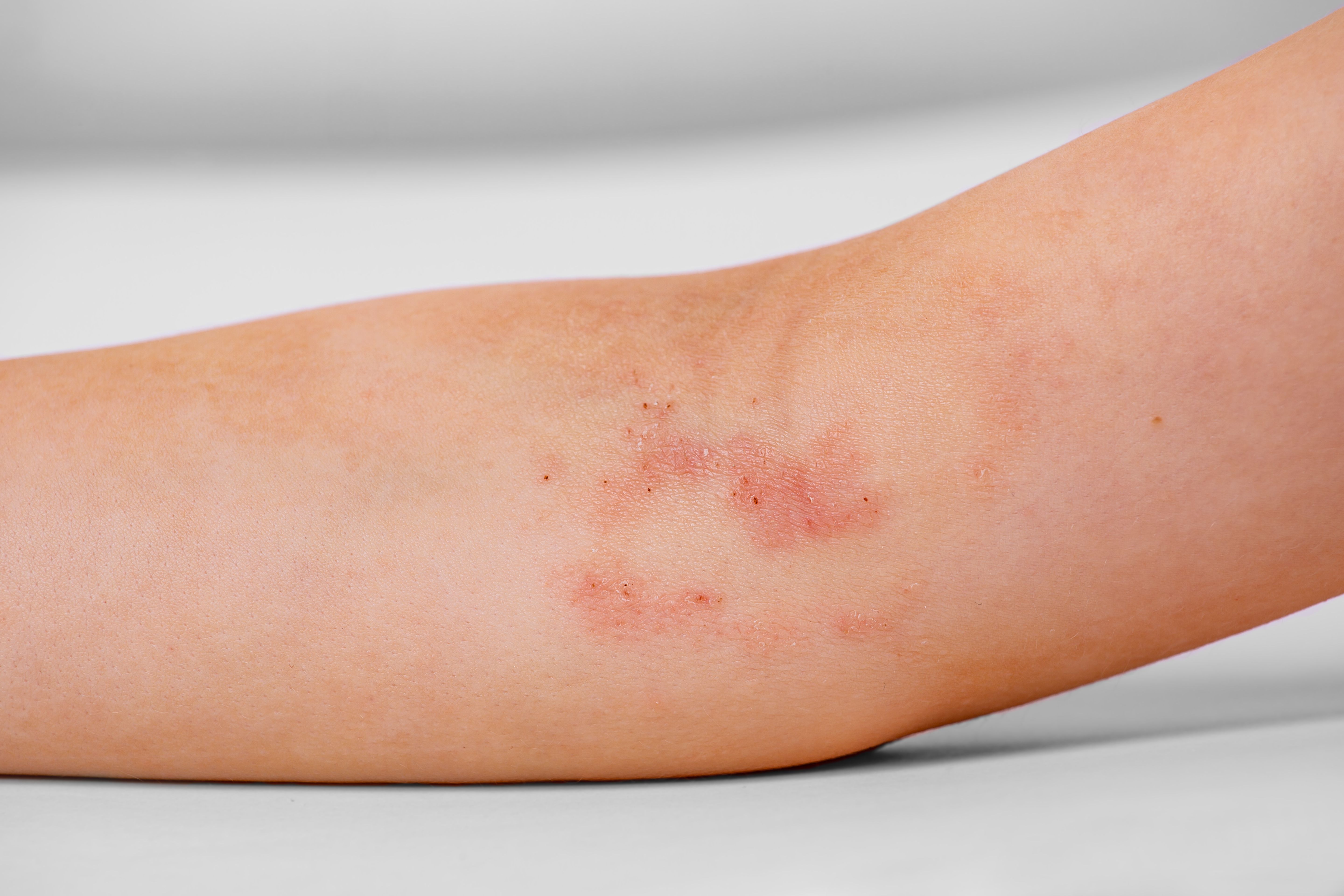
New data presented at the European Academy of Dermatology and Venereology (EADV) Congress revealed lebrikizumab (Eli Lilly and Company) demonstrated clinical improvements in combination with topical corticosteroids (TCS) for adolescent and adult patients with moderate-to-severe atopic dermatitis (AD) that were not adequately controlled with cyclosporine (or for those whom cyclosporine was not medically advisable), according to a recent news release from Almirall.1
The data presented was from the phase 3 ADvantage study (NCT05149313), a randomized, double-blind, placebo-controlled clinical study to evaluate safety and efficacy of lebrikizumab in combination with TCS. The trial included 331 adolescents and adults aged 12 years and up that were assessed over 16 weeks. Safety was consistent with the lebrikizumab’s known safety profile.1,2
Depth of response data was also presented in patients that took part in the Advocate 1 (NCT04146363) and Advocate 2 (NCT04178967) phase 3 studies that took place over 52 weeks. Deep responses were defined as total skin clearance (Investigator’s Global Assessment [IGA], Eczema Area and Severity Index [EASI] 100, and itch relief [NRS 0,1]). Deep responses were achieved in 20% and 31% of patients in ADvocate 1 and ADvocate 2, respectively, by week 16 and were maintained or increased through week 52.1
Long-term clinically meaningful responses were observed in patients that help achieve disease control. Eighty-four percent of patients in the ADvocate 1 and 2 studies that responded to lebrikizumab at week 16 achieved a “clinically meaningful response in at least [1] domain of the disease (mild signs, symptoms, or quality of life impact),” at the 52-week mark, according to Almirall. Over 57% demonstrated response across all 3 domains.1
A significantly higher proportion of participants treated with lebrikizumab achieved EASI of 7 or less (mild) and EASI of 1 or less (clear/almost clear) at week 16 compared to placebo, a post-hoc analysis of the ADvocate 1 and Advocate 2 trials revealed.1
Regardless of baseline severity, the analysis revealed over 50% of those treated with lebrikizumab 250 mg every other week on nontherapy for 16 weeks achieved an EASI score indicating mild AD. An EASI score indicating clear or almost clear was achieved in approximately 20% of participants.1
Eli Lilly and Company owns exclusive rights for the development and commercialization of lebrikizumab in the United States and the rest of the world, not including Europe, where Almirall has licensed the rights for development and commercialization.1
References:
1. Almirall's lebrikizumab improves signs and symptoms of moderate-to-severe atopic dermatitis (AD) in patients inadequately controlled with or ineligible for cyclosporine. Almirall. Press release. October 13, 2023. Accessed October 17, 2023. https://www.almirall.com/newsroom/news/almiralls-lebrikizumab-improves-signs-and-symptoms-of-moderate-to-severe-atopic-dermatitis-ad-in-patients-inadequately-controlled-with-or-ineligible-for-cyclosporine
2. A study of lebrikizumab in combination with topical corticosteroids in participants having atopic dermatitis (AD) that are not adequately controlled or non-eligible for cyclosporine. Clinicaltrials.gov. Updated January 19, 2023. Accessed October 17, 2023. https://www.clinicaltrials.gov/study/NCT05149313