NICU Micropreemies: How do they fare?
When preemies weighing less than 800 g survive-as more and more of them do-parents want to know what quality of life their child can expect. The answer is that, despite the risk of neurodevelopmental disabilities difficulties are rarely severe, resources are available for overcoming problems, and more of these children will live a normal, productive life.
Ethan was born at 25 weeks' gestation weighing 730 g. Apgar scores were 2, 5, and 6 at 1, 5, and 10 minutes, respectively. Four doses of surfactant were administered. He remained on a ventilator for three weeks and continuous positive airway pressure for an additional seven weeks.
Complications included blood culture–positive bacterial and fungal sepsis and a bilateral germinal matrix hemorrhage (Grade I) with blood in the right lateral ventricle (Grade II). He received total parenteral nutrition for three weeks, followed by enteral feedings with 27 kcal/oz formula while fluid restricted for chronic lung disease. He also underwent laser treatment for retinopathy of prematurity with good results.
Ethan spent three months in the hospital. He was discharged on low-flow oxygen to maintain oxygen saturation in the 90% range, with instructions to return to the neonatal follow-up clinic in one month.
Ethan's last follow-up was at chronological age 2 years, 8 months. At that time, he was generally healthy, wore glasses for strabismus correction, and received daily inhaled steroid treatments and nebulized albuterol as needed. His height, weight, and head circumference were below the third percentile, even for adjusted age. He had been evaluated for possible growth hormone treatment. His neurologic examination was normal, and his developmental skills were appropriate for his age.
Pediatricians who care for babies like Ethan are faced with some pressing questions:
- At the time of birth, what can you tell parents about the child's future quality of life and their ability to meet his needs?
- How would his clinical course change those predictions?
- Should a normal exam as a toddler (except for small stature, in Ethan's case) reassure the parents that he will be "normal?"
The following review addresses these questions.
*The REVOLUTION in VIABILITY
When I was a medical student and pediatric resident in the mid-1970s, I witnessed the emergence of modern neonatal intensive care that followed a significant change in respirator technology. In 1974, premature infants were often ventilated with bulky Emerson or Bennett MA-1 machines, designed for adult lungs. Delivering a tiny volume of air to a 1,200-g premature infant and overcoming the huge dead space of long, large-bore tubing with these devices was a challenge we often could not meet. A scant two years later, however, the Baby Bird infant ventilator revolutionized treatment of respiratory distress syndrome and dramatically improved survival rates for premature babies.
The 1980s and 1990s brought additional significant advances, including the use of surfactant and routine antenatal steroids, that pushed the limits of viability to 24 to 25 weeks' gestation. Recent reports of the short- and long-term outcomes of these tiny infants provide the basis of judging the efficacy of neonatal intensive care for the smallest newborns, giving guidance to parents in the early decision-making period and anticipating the financial, educational, social, and other resources they might require.
*Defining TERMS
As ever-smaller premature infants have survived, the terminology we use to describe them has evolved. Now, "low birth weight" (LBW) refers to newborns weighing less than 2,500 g; "very low birth weight," (VLBW) to less than 1,500 g; and "extremely low birth weight," to less than 1,000 g. The term "micropreemie" was coined for infants less than 800 g.
Birth weights must be correlated with gestational age to determine whether the infant is appropriately sized. However, much of the neonatal follow-up literature is based on birth weight or gestational age alone, and does not separate out infants who are small for gestational age because of intrauterine growth retardation, which confers independent risk for future growth and developmental problems. This review focuses on outcomes for the micropreemie infant.
*Trends in SURVIVAL vs. DISABILITY
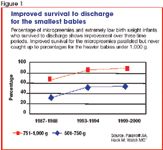
The National Institutes of Health (NIH)-sponsored Neonatal Research Network has kept data on mortality, morbidity, and outcomes of premature infants since 1986. Across three time periods, the percentage of extremely low birth weight infants who survive to discharge from the neonatal intensive care unit (NICU) has risen steadily (Figure 1).1 The improvement in survival for the micropreemies paralleled, but never caught up to, that for the heavier infants under 1,000 g, reaching 55% by the year 2000. Perhaps accounting for this improvement was a marked increase in the use of antenatal steroids, cesarean sections, and the introduction of surfactant.1
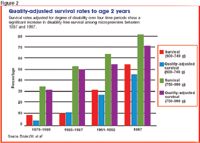
Researchers in Australia have adjusted survival rates for premature infants by their degree of disability. The greater the number or severity of disabilities among survivors, the lower the adjusted rate for that time period (Figure 2). Between 1979 and 1987, micropreemies showed no increase in disability-free survival rates. But thereafter to 1997, dramatic improvement occurred, suggesting that more micropreemies were not only surviving but doing so without a significant increase in severe sensory, physical, or developmental morbidity.2
*$$$$$ COSTS
Data from California show that the hospital cost of caring for a 25-week-gestation infant averaged $203,000, not including physicians' fees. Cost dropped to $46,000 for a 30-week-gestation infant. The cost of care for a normal newborn is about $1,000.3 Additional costs accrue after discharge for medications, specialized formulas, subspecialty medical visits, therapeutic services, and transportation. Some of these costs are not covered or only partially covered by insurance, which places a heavy financial burden on families. Parents may have to work less or even quit their jobs to be at their infant's bedside in the NICU and provide for special care required when the infant comes home.
Much has been written about and by parents regarding the emotional cost of having an extremely premature infant-or more than one, as is often the case with multiple births. [Editor's note: For more on this topic, see "Questions and answers about multiple births" in the January 2007 issue www.contemporarypediatrics.com/.]
Maternal depression and anxiety can continue for years after birth.4 When developmental outcomes are unfavorable, the severity of maternal depression is greater.
*Neurodevelopmental OUTCOMES
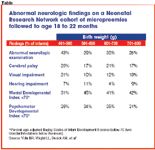
One hundred grams can make a significant difference in outcome. Among micropreemies weighing less than 800 g at birth, about 40% of infants in the 400- to 500-g range have abnormal neurologic examinations at 20-month follow-up, and about 30% have cerebral palsy (see Table).5 In addition, 30% to 35% have mental or psychomotor indices below 70 (two standard deviations below the mean) on the Bayley Scales of Infant Development. Rates of these problems decrease significantly at heavier weights, but remain fairly steady between 500 and 800 g.
When follow-up is extended into school age, the overall disability rate increases. The EPICure Study included all infants of 25 weeks' gestation or less born in 1995 throughout the United Kingdom and Ireland. Disability rates were obtained at 6-year follow-up, based on IQ scores as well as neurologic assessment. Overall, 80% of the children had some disability: mild in 34%, moderate in 24%, and severe in 22%. Only severe disability at 30 months of age predicted developmental problems at 6 years.6
The advantage for survival of female micropreemies in the neonatal period persists: Girls consistently have higher cognitive processing and achievement scores than boys.7,8
Researchers in Victoria, Australia found certain combinations of risk factors to be most predictive of major neurosensory disability: a grade III or IV intraperiventricular hemorrhage, cystic periventricular leukomalacia, surgery (of any type), and use of postnatal steroids for chronic lung disease. If none of these risk factors were present, the likelihood of severe disability was zero. But if an infant had all four, the chance of severe disability was 100%.9
Among micropreemies with intelligence in the normal range, a pattern of learning disabilities has emerged. These children tend to have more difficulty with perceptual performance tasks, such as block design, digit symbols, and arithmetic. Their performance on verbal tasks, including reading, spelling, and vocabulary (Figure 3), is less impaired.10
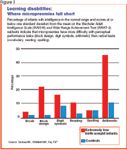
From these studies, we can conclude that roughly 20% of micropreemies show severe disabilities, 20% are moderately disabled, and 20% to 30% have mild disabilities. Generally, NICU follow-up programs identify children with moderate to severe disabilities. Unfortunately, monitoring is typically discontinued well before school entry, the time when learning problems emerge. It is important that parents understand the risks of school difficulties for their child, so that problems may be identified and remediated as early as possible to prevent frustration, poor self-image, and school failure.
Similarly, attention deficit hyperactivity disorder (ADHD) appears to be common among former micropreemies.11 The combination of learning disabilities and ADHD may account for a portion of the observed high rates of behavior problems such as depression, anxiety, social incompetence, and oppositionality in these children. Adolescents who were micropreemies rate themselves lower in scholastic, athletic, job, and even romantic competencies.10 Compared to parents of normal birth weight infants, parents of former micropreemies report more functional limitations, dependency needs, and use of extra services for their children.
Despite this rather negative view, micropreemies without major disabilities appear to do quite well in the long run. They have the same rates as their peers of high school graduation, postgraduate education, employment, independent living, marriage or cohabitation, and parenthood.12 Perhaps these positive long-term outcomes are what are important for parents to hear in the NICU and in early follow-up, despite problems along the way.
*Brain development, VULNERABILITY, and INJURY
Ever since Papile devised a grading system for intraventricular hemorrhage (IVH) in the 1960s, poor outcomes (mostly motor) have been attributed to discernable lesions observed on routine head ultrasound. A different kind of nonhemorrhagic lesion, periventricular leukomalacia, was found to be highly predictive of cerebral palsy.13 These white matter lesions, even the most extensive, are not inevitably associated with cognitive deficits, suggesting sparing of the cortex.14
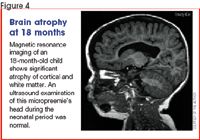
It has become clear, however, that a large percentage of micropreemies with normal head ultrasounds in the NICU have significant cognitive deficits and learning problems even with normal intelligence (Figure 4).15 Recent studies using sophisticated brain imaging techniques reveal more subtle abnormalities of white and gray matter, such as reduced gray matter and deep nuclear volume and diffuse white matter atrophy.16 These abnormalities may result from immature vascular supply and regulation in watershed areas of the white matter.
It also appears that the oligodendroglial precursor cells (responsible for myelin formation) are particularly vulnerable to ischemia in micropreemies. Gray matter abnormalities are not destructive but may result from sublethal injury to or axonal disruption in the corresponding white matter.17 This is an active area of research in which better understanding may lead to preventive interventions.
* GROWTH
Micropreemies typically show very poor growth in the first few weeks and months of life, because nutrition is a major challenge. Gastrointestinal intolerance, lung disease, and infections are just a few factors that complicate the task of providing sufficient calories during a period of high demand. As seen in Figure 5, these babies drop well below their expected growth parameters with a nadir at about term. Thereafter, they begin their "catch-up growth," provided they are well enough to tolerate high formula volumes or enhanced caloric density.18

Infants with chronic lung disease (in which too much fluid can exacerbate symptoms, despite diuretics) or gastroesophageal reflux (in which minimal increases in amount ingested exaggerate losses with emesis) can remain suboptimally nourished for many months. Medications, quantity and concentration of formula, and specialized feeding techniques must be carefully orchestrated with the help of multiple specialists to maintain good nutrition during this important growth stage. Prolonged periods of poor growth likely have both short- and long-term neurodevelopmental ill effects.19
Note in Figure 5 that micropreemies with intrauterine growth retardation (small for gestational age) follow a similar growth pattern but remain smaller in all growth parameters. Significant growth retardation-height anticipated to be below the fifth percentile in adulthood-may be an indication for treatment with growth hormone.20 If a child remains below the fifth percentile by 3 years of age, referral to an endocrinologist should be discussed with the parents. Evidence suggests that micropreemies who were not growth retarded at birth demonstrate a second catch-up phase during adolescence.21
*Follow-up CARE
Infants with the highest likelihood of severe disability can be identified before hospital discharge by the presence of risk factors such as an extensive IVH, chronic lung disease, or prolonged hospitalization. Hospital staff have the opportunity to discuss these risks with parents and to make appropriate referrals.
Most NICUs have follow-up clinics where residual medical problems (such as a persistent supplemental oxygen requirements or feeding problems) and developmental progress are monitored in collaboration with the infant's primary care provider. Unfortunately, funding constraints prevent many of these follow-up programs from continuing monitoring into school age.
Our clinic follows infants for 12 to 18 months on average, until medical problems have resolved and we feel confident that the baby has no moderate to severe developmental problems that would necessitate a referral for early intervention (EI). Babies with significant developmental disabilities are referred to a developmental or rehabilitation clinic where a full array of interdisciplinary services is available, including nutrition, orthotics, audiology, and neuropsychology.
Once micropreemies are discharged from the follow-up clinic, continuing developmental surveillance becomes the responsibility of the child's primary care provider. Parents should be alerted to the roughly 30% to 40% chance that their child may have some learning problems detectable at school entry, so that the child's adaptation and progress at school can be closely monitored, and further evaluation undertaken, if indicated.
*What EI can accomplish
The term "early intervention" refers to government-funded, community-based support systems provided for infants and toddlers with or at risk for developmental disabilities. In most states, micropreemies are considered at risk, and therefore qualify for services. Services may include monitoring developmental progress by a parent-infant educator, coordination of complicated care, parent support groups, and direct physical, occupational, or speech therapy. Cutbacks in federal and state funding in recent years have forced EI programs to rely on medical insurance reimbursement to sustain their services. As a result, parents may be subjected to co-pays, deductibles, and limited choices of service providers, whose pediatric training and experience may be limited.
Whether EI "works" or not depends on many variables, including the nature and severity of the child's developmental challenges, the quality of the services provided, and the outcomes that are desired. Studies have shown repeatedly that EI does not prevent cerebral palsy in babies who have significant white matter injury or speed up attainment of gross motor milestones in an infant with hypotonia.22,23 In contrast, EI can successfully facilitate independent mobility in an infant who has been restricted by technological dependence for many months.
Normalization of experience for the infant should be the primary goal of EI and has the best chance of accomplishing positive outcomes. Returning a feeding-tube–dependent infant to oral feedings, making respiratory support equipment as mobile as possible, and establishing parent-infant bonding after prolonged hospitalization are examples of normalization. Creativity, flexibility, and teamwork-and sometimes heroic efforts-are important components of this EI model.
While most EI programs focus on short-term goals, some evidence exists that early education programs for young children at biological risk have long-term benefits on school achievement, language, and even reduction of risk behaviors during adolescence.24 Such positive results are most likely to occur if children at risk remain in EI programs and transition to preschool special education or Head Start programs at 3 years of age. Even when early therapy services are discontinued before 3 years, given the high rate of learning difficulties among former micropreemies, a preschool experience of some sort is highly recommended to enhance social, behavioral, and academic preparation for kindergarten.
***Recommendations
Many more micropreemies are surviving. Fortunately, they do not have very much higher rates of severe disability than larger premature infants. Severe and some moderate developmental problems are usually anticipated before discharge from the NICU or discovered through NICU follow-up clinics, so that appropriate referrals can be made to developmental specialists and early intervention services. However, these children have a substantial risk of learning, attentional, and behavioral problems, which may only become apparent as they progress through school.
By 3 years of age, all former micropreemies should have participated in a preschool program, whether private, public, or Head Start. This two- to three-year experience provides a strong foundation in preacademic skills, group social interaction, adaptation to set routines, practice with focused attention, and appropriate intervention for emerging behavioral difficulties.
If a child manifests learning problems during preschool or at school entry, he or she should be referred to the public school system for a full evaluation under the Individuals with Disabilities Education Act (IDEA). This federal law mandates a free and appropriate individualized education in the least restrictive environment for all children. If a child does not qualify for services under IDEA, other programs exist that can assist those with difficulties in specific subjects such as reading or mathematics.
Parents need support during the transition from NICU to home, not only for medical issues but also for their concerns about developmental outcomes. If the baby survives the first days and weeks and does not have major complications, parents are often told their baby is "fine." They may interpret this to mean "guaranteed normal in every way." They need to be advised about the full range of outcomes, and formulate tentative plans for what to do if problems become evident at any time. This does mean fixating on possible negative outcomes but simply providing the usual supportive anticipatory guidance.
*What do we tell ETHAN'S PARENTS?
Encounters with patients like Ethan and his parents provide gratifying experiences for pediatricians. By the time the child is 2 years of age, we can confidently tell parents that worries about additional major disability are past, but learning problems are still a possibility. In the meantime, a preschool experience would be valuable. When the child enters school, parents and teachers should be alert to early signs of learning difficulties. A learning clinic or the school itself can provide in-depth evaluations if warranted. Despite the cost, long hospitalization, and emotional burden, to his parents, Ethan is priceless.
Accreditation
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of CME2, Inc. ("cme2") and Contemporary Pediatrics. cme2 is accredited by the ACCME to provide continuing medical education for physicians.
cme2 designates this educational activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Target audience: Pediatricians and primary care physicians
Educational objectives
- -Anticipate the developmental and behavioral problems micropreemie infants demonstrate in later childhood
- -Distinguish the growth patterns between appropriate and small-for-gestational-age micropreemie infants
- -Relate the developmental disabilities of micropreemie infants to specific white and gray matter abnormalities
- -Know the changes in mortality and morbidity occurring over the past 25 years among micropreemie infants and the reasons for these changes
To earn CME credit for this activity
Participants should study the article and log on to www.contemporarypediatrics.com/, where they must pass a post-test and complete an online evaluation of the CME activity. After passing the post-test and completing the online evaluation, a CME certificate will be e-mailed to them. The release date for this activity is February 1, 2007. The expiration date is February 1, 2008.
Disclosures
Editors Toby Hindin, Jeff Ryan, Karen Bardossi, and Karen Woldman disclose that they do not have any financial relationships with any manufacturer in this area of medicine.
Manuscript reviewers disclose that they do not have any financial relationships with any manufacturer in this area of medicine.
Author James A. Blackman, MD, MPH discloses that he does not have any financial relationships with any manufacturer in this area of medicine.
Resolution of conflict of interest
cme2 has implemented a process to resolve conflicts of interest for each continuing medical education activity, to help ensure content validity, independence, fair balance, and that the content is aligned with the interest of the public. Conflicts, if any, are resolved through a peer review process.
Unapproved/off-label use discussion
Faculty may discuss information about pharmaceutical agents, devices, or diagnostic products that are outside of FDA-approved labeling. This information is intended solely for CME and is not intended to promote off-label use of these medications. If you have questions, contact the medical affairs department of the manufacturer for the most recent prescribing information. Faculty are required to disclose any off-label discussion.
LEARNING OBJECTIVES
After reviewing this article, the physician should be able to:
- Anticipate the developmental and behavioral problems micropreemie infants demonstrate in later childhood
- Distinguish the growth patterns between appropriate and small-for-gestational-age micropreemie infants
- Relate the developmental disabilities of micropreemie infants to specific white and gray matter abnormalities
- Know the changes in mortality and morbidity occurring over the past 25 years among micropreemie infants and the reasons for these changes
DR. BLACKMAN is professor of pediatrics and head, division of developmental pediatrics, Kluge Children's Rehabilitation Center, Children's Medical Center, University of Virginia, Charlottesville.
REFERENCES
1. Fanaroff AA, Hack M, Walsh MC: The NICHD Neonatal Research Network: Changes in practice and outcomes during the first 15 years. Semin Perinatol 2003;27:281
2. Doyle LW, Victorian Infant Collaborative Study Group: Evaluation of neonatal intensive care for extremely low birth weight infants in Victoria over two decades: I. Effectiveness. Pediatrics 2004;113:505
3. Gilbert WM, Nesbitt TS, Danielsen B: The cost of prematurity: Quantification by gestational age and birth weight. Obstet Gynecol 2003;102:488
4. Singer LT, Salvator A, Guo S, et al: Maternal psychological distress and parenting stress after the birth of a very-low-birth-weight infant. JAMA 1999;281:799
5. Vohr BR, Wright LL, Dusick AM, et al: Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institutes of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 2000;105:1216
6. Wood NS, Marlow N, Costeloe K, et al: Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med 2000;343:378
7. Morse SB, Wu SS, Ma C, et al: Racial and gender differences in the viability of extremely low birth weight infants: A population-based study. Pediatrics 2006;117:e106
8. Marlow N, Wolke D, Bracewell MA, et al: Neurologic and developmental disability at 6 years of age after extremely preterm birth. N Engl J Med 2005;352:9
9. Doyle LW, Casalaz D: Victorian Infant Collaborative Study Group: Outcome at 14 years of extremely low birthweight infants: a regional study. Arch Dis Child Fetal Neonatal Ed 2001;85:F159
10. Grunau RE, Whitfield MF, Fay TB: Psychosocial and academic characteristics of extremely low birth weight (?800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics 2004;114:e725
11. Anderson P, Doyle LW, Victorian Infant Collaborative Study Group: Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003;289:3264
12. Saigal S, Stoskopf B, Streiner D, et al: Transition of extremely low-birth-weight infants from adolescence to young adulthood: Comparison with normal birth-weight controls. JAMA 2006;295:667
13. de Vries LS, Van Haastert I-L, Rademaker KJ, et al: Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr 2004;144:815
14. Blackman JA, McGuinness GA, Bale JF Jr., et al: Large postnatally acquired porencephalic cysts: unexpected developmental outcomes. J Child Neurol 1991;6:58
15. Laptook AR, O'Shea TM, Shankaran S, et al: Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound. Prevalence and antecedents. Pediatrics 2005;115:673
16. Inder TE, Warfield SK, Wang H, et al: Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005;115:286
17. Volpe JJ: Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics 2005;116:221
18. Jordan IM, Robert A, Francart J, et al: Growth in extremely low birth weight infants up to three years. Biol Neonate 2005;88:57
19. Latal-Hajnal B, von Siebenthal K, Kovari H, et al: Postnatal growth in VLBW infants. Significant association with neurodevelopmental outcome. J Pediatr 2003;143:163
20. Czernichow P, Fjellestad-Paulsen A: Growth hormone in the treatment of short stature in young children with intrauterine growth retardation. Horm Res 1998;49:2(23)
21. Hirata T, Bosque E: When they grow up: The growth of extremely low birth weight (?1000 gm) infants at adolescence. J Pediatr 1998;132:1033
22. Palmer FB, Shapiro BK, Wachtel RC, et al: The effects of physical therapy on cerebral palsy. A controlled trial in infants with spastic displegia. N Engl J Med 1988;318:803
23. Rothberg AD, Goodman M, Jacklin LA, et al: Six-year follow-up of early physiotherapy intervention in very low birth weight infants. Pediatrics 1991;88:547
24. McCormick MC, Brooks-Gunn J, Buka SL, et al: Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics 2006;117:771