Otitis media with effusion (OME) and child development: Rethinking management
A CME article on otitis media and MEE and its implications on hearing loss in children.

This article reviews the rationale for the recommendations in the guideline, and provides an algorithm for management.
Profile of a familiar foe
OME, whether as the aftermath of AOM or arising de novo as a complication of upper respiratory infection, often predisposes to renewed or new middle-ear infection. A hallmark both of AOM and OME is the presence of middle-ear effusion (MEE). Episodes of AOM are characterized by the onset of acute symptoms: ear pain, ear tugging, irritability, and/or fever (whose duration is measured in days). In contrast, OME, especially in infants and young children, usually does not present with noticeable symptoms. Approximately 75% to 90% of OME episodes resolve spontaneously within three months.2 Rarely, cases remain unresolved after a year or longer.
Diagnosis of MEE
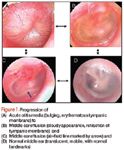
OME and treatment
Antimicrobial treatment may offer short-term improvement in children with OME.4 The only treatment known to be lastingly effective is myringotomy with tympanostomy-tube insertion (M&T).5
M&T immediately relieves MEE by allowing suction and drainage of accumulated fluid and ventilation of the middle ear via the tympanostomy tube. However, common complications of M&T may offset its effectiveness. These include obstruction of the tube lumen, secondary infection with tube otorrhea, and premature extrusion. Sequelae following tubal extrusion are also not uncommon. In various series, residual perforation of the eardrum has been noted in about 2% of ears; myringosclerosis (whitish calcific plaques on the tympanic membrane) in 30% to 50% of ears; segmental atrophy (localized area of thinning of the tympanic membrane), atelectasis, retraction, or retraction pocket in 25% to 50% of ears; and cholesteatoma in 0.7% of ears.6 In the near term, most of these changes are not accompanied by reductions in hearing acuity. Whether hearing loss may develop or become exaggerated later as a consequence of tube insertion remains speculative, and concerning.
MEE and hearing acuity
Both AOM and OME are usually accompanied by conductive hearing loss of variable degree that persists until the underlying condition has resolved. During the first few years of life, auditory thresholds in normal children, as in children with MEE, depend somewhat on age and testing method. These thresholds tend to be higher the younger the child is. In normal children less than 3 years of age, the mean four-frequency pure-tone average (PTA) hearing threshold is 15 to 20 decibels hearing level (dB HL). This increases to 20 to 35 dB HL when MEE is present. Similarly, the mean speech awareness threshold (SAT) in young children is about 15 dB HL, and increases to about 20 to 25 dB HL when MEE is present.7
The impact of conductive hearing loss on speech perception depends on both the degree of loss and the intensity/loudness of the speech. The sound level produced by conversational speech averages about 60 dB at a distance of one meter, but wide variations exist depending on speech volume. If, for instance, a child's hearing threshold is elevated to 30 dB HL, he or she would generally be able to hear conversational speech. On the other hand, if the speech volume is low or spoken in a whisper, with a sound level of 30 dB, the child may be unable to hear it.
When sound levels are near the borderline of detection, distortions and/or partial perceptions of speech are more likely than when sound levels are in the clearly detectable range. In addition, infants have been shown to have higher thresholds than adults for discriminating between certain speech sounds, and thus may require greater stimulus intensity for satisfactory speech perception.8
Mounting concern over development
Beginning in the late 1960s, many professionals became concerned that persistent MEE in the first few years of life could cause long-term adverse effects on a child's development. Their reasoning began with consideration of two sets of circumstances. First, children with moderate to severe, permanent sensorineural hearing loss have delays and disorders in speech and language,9 and related cognitive and behavioral problems. Second, the peak prevalence of otitis media with effusion, with its associated conductive hearing loss, occurs during the first three years of life, a period of rapid development in speech and language. If the first three years of life constitute a critical period for speech and language development, then it seemed reasonable to presume that disruptions in development during that period could ultimately lead to permanent disorders in these domains.
Early studies concerning possible relationships gave variable results, with many of the studies indeed showing associations between persistent or recurrent early-life otitis media and later developmental problems. However, most of these studies had important weaknesses in their methodology. Notably, all of these studies were strictly associational in nature, ie, they considered whether children with more episodes of MEE early in life ended up with less favorable developmental outcomes, or conversely, whether children with less favorable developmental outcomes had experienced more MEE early in life. Such associational studies warrant no inferences concerning possible cause-and-effect relationships.10
The search for answers
In 1991, we embarked on the Children's Hospital of Pittsburgh Child Development/Otitis Media study.1 The primary goal of this work was to determine whether persistent MEE in children's first three years of life would actually cause long-term developmental delays or disorders.
We excluded children from the study who had biological and/or social risk factors that can compromise health and development,11 such as prematurity and placement in foster care. To determine causality, we would first need to identify a large population of young children with persistent MEE, then divide them randomly into two equivalent groups-one of which would thereafter have relatively little MEE, and the other, relatively more MEE. To shorten the duration of MEE in the one group and restore their hearing to normal, we planned to subject those children to M&T. If differences in developmental outcomes between the two groups were found later on, one could confidently infer that those differences were attributable to differences in the amount of MEE the two groups had experienced.
We enrolled 6,350 children at less than 2 months of age. We monitored all study participants closely using pneumatic otoscopy, supplemented often by tympanometry as a means to determine the duration of MEE that each child experienced. In children who developed MEE, we assessed hearing acuity periodically. Children were eligible for the randomized clinical trial if they developed MEE within the first three years of life, if it appeared substantial in degree, and if it persisted for 90 days in the case of bilateral effusion and 135 days for unilateral effusion. We also developed specified, longer periods of time for intermittent bilateral or unilateral effusion.
When children crossed that threshold, we randomly assigned them to either undergo prompt M&T (the early-treatment group) or be observed for an additional six months, with M&T available if MEE were to continue to persist (the delayed-treatment group).10 A total of 429 children were randomized. We then compared the two groups developmentally when they reached ages 3, 4, 6, and 9 to 11 years.