Pediatric clinical trials lead to new drugs, updates in 2019
The year 2019 has seen major advances for pediatric therapeutics in the quest to identify better medicines for children. Here is a summary of what is new from these last months.
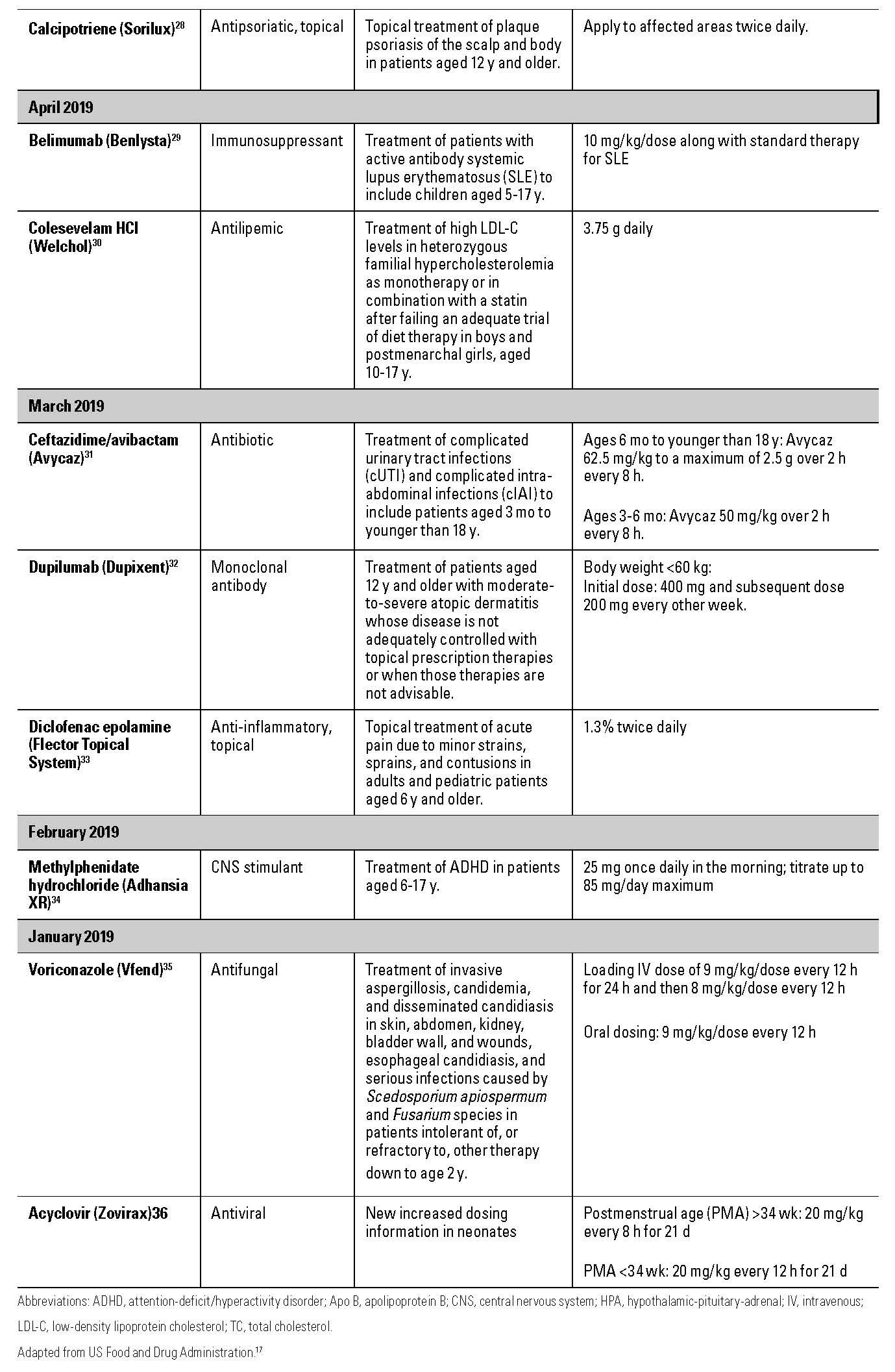
Table 1
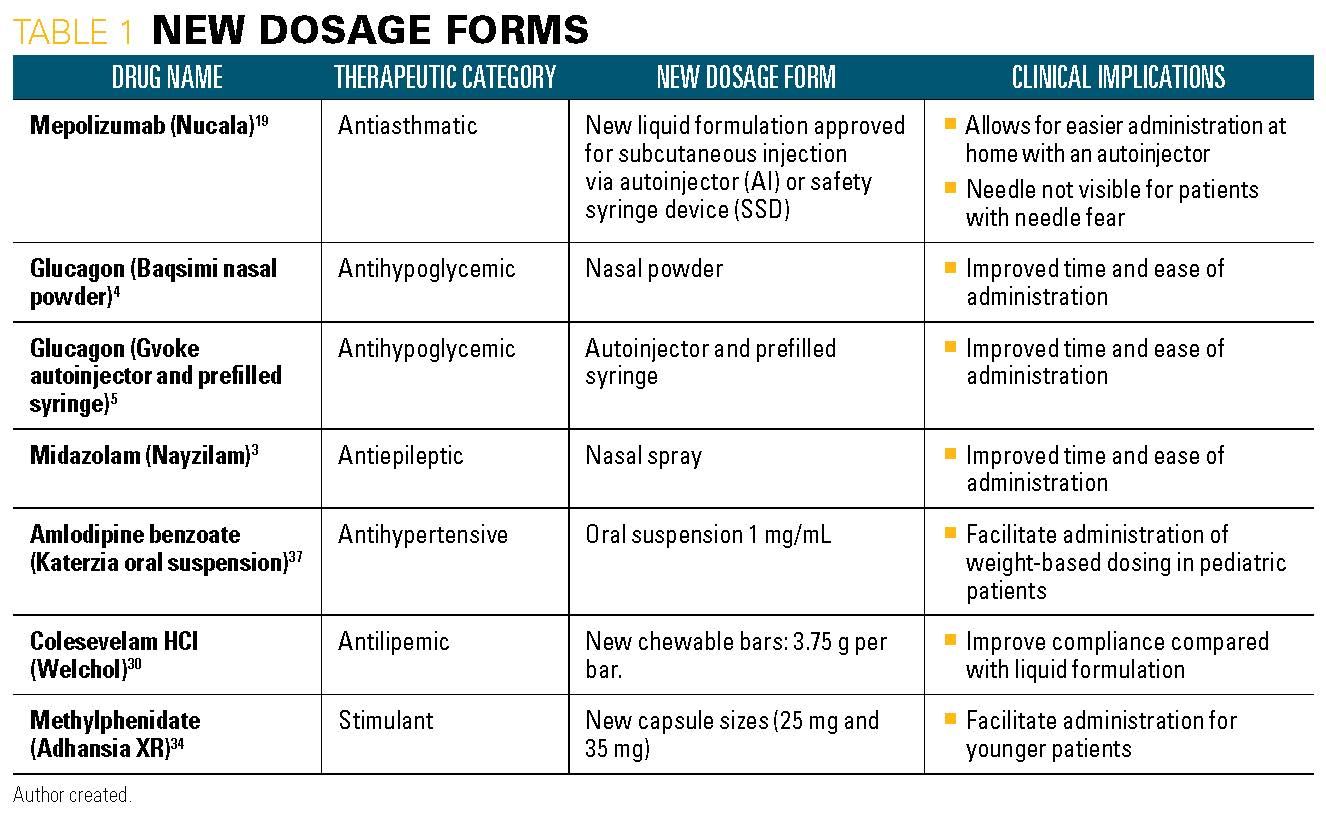
Table 2
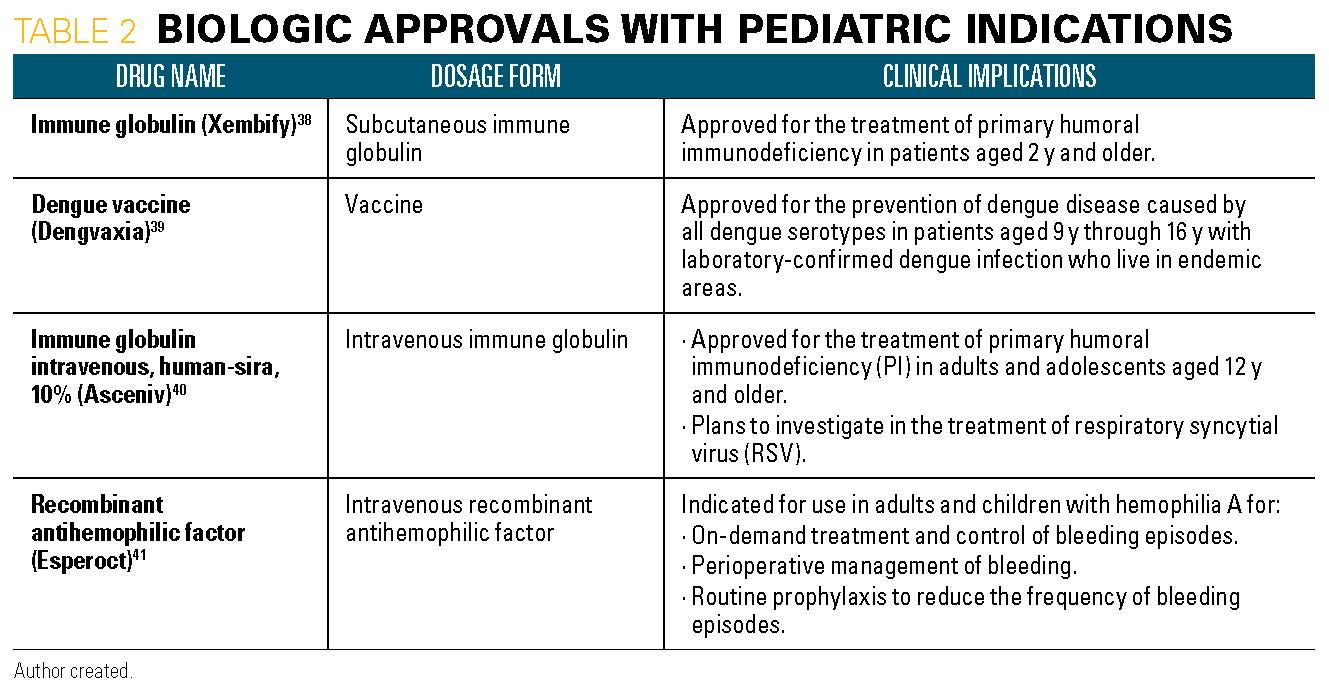
Table 3, part 1
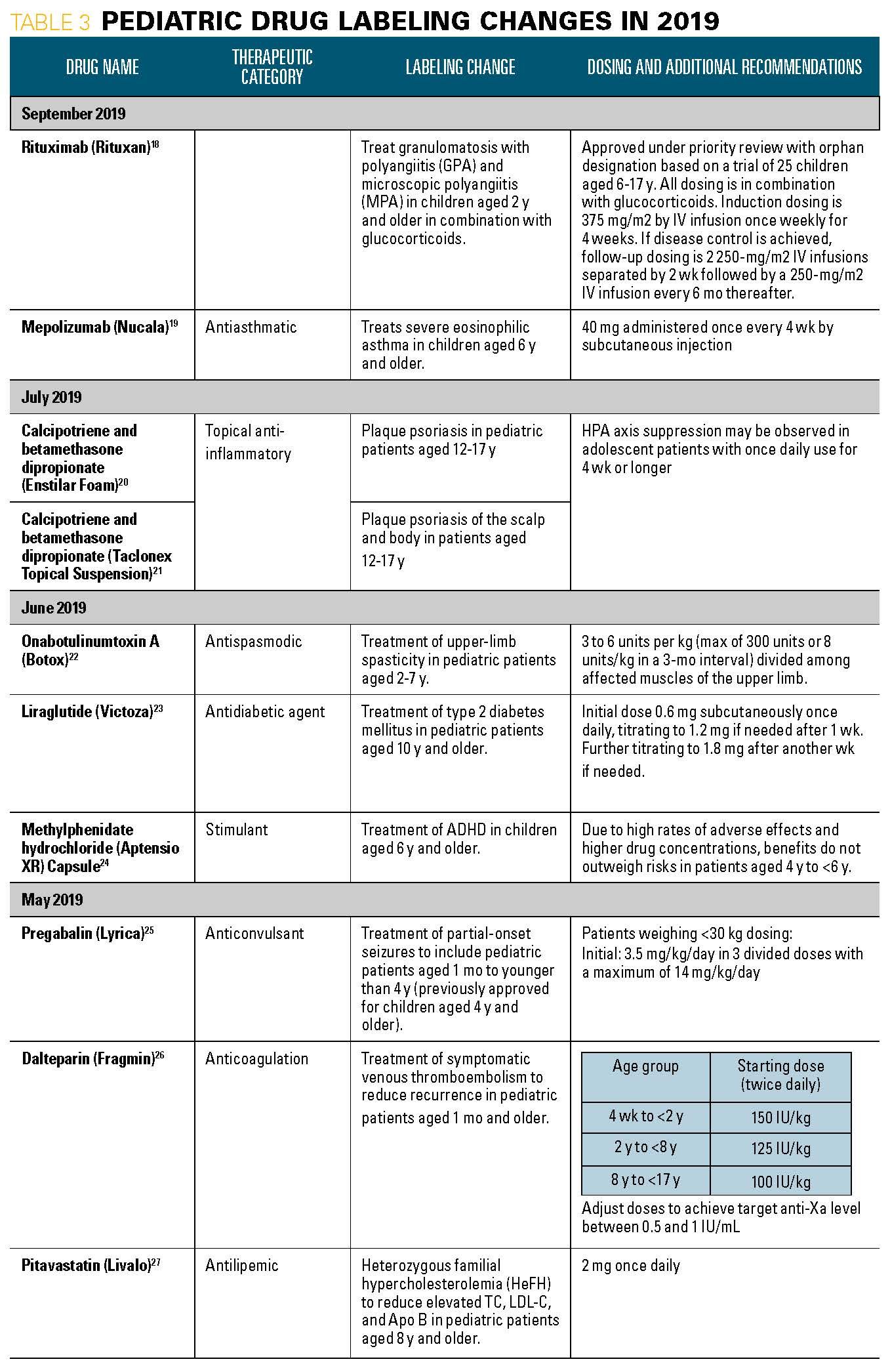
Table 3, part 2
Off-label use of medication in pediatric patients has varied from 25% to 85%.1,2 Federal legislation including the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA) have increased the number of clinical trials conducted in children and led to expanded labeling for these patients. This article summarizes recent (November 2018 to September 2019) labeling changes, new dosage forms, and new drugs that have potential for use in children.
Table 1 includes information on new dosage forms and Table 2 lists new biologics with pediatric indications. Table 3 lists labeling changes to existing drugs.
New dosage forms
NAYZILAM (MIDAZOLAM NASAL SPRAY)
In May 2019, the US Food and Drug Administration (FDA) approved the first nasal spray for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern in patients aged 12 years and older with epilepsy.3 The nasal spray comes in a single dose of 5 mg of midazolam, and each package contains 2 spray units. The sprayer should not be primed prior to use as this will cause loss of the dose. The caregiver should administer a dose into one nostril, and if the seizure continues beyond 10 minutes, a second dose with a new spray unit may be given.
Many patients with refractory epilepsy have used intranasal midazolam compounded in compounding pharmacies; unlike Nayzilam, these products typically have multiple doses. Nayzilam is a single dose, similar to Diastat (rectal diazepam gel) that has been on the market since 1997.
BAQSIMI (GLUCAGON NASAL SPRAY) AND GVOKE (GLUCAGON AUTOINJECTOR AND PREFILLED SYRINGE)
Glucagon has been available in a subcutaneous kit for many years and has been the mainstay of emergency treatment for hypoglycemic emergencies. Recently, the FDA approved Baqsimi nasal spray (July 2019)4 and the Gvoke HypoPen and Gvoke PFS (September 2019),5 both of which will provide glucagon in a ready-to-use form. Glucagon in liquid form was previously not stable at room temperature and the traditional kit requires the user to reconstitute the powder with a diluent using a needle and syringe. These steps can be intimidating and difficult, especially for a user who is experiencing a hypoglycemic emergency.
Baqsimi is available as a 3-mg intranasal device that contains one dose. The 3-mg dose is indicated for ages 4 years and older and is administered into one nostril. If no response is seen in 15 minutes, a second dose may be administered. Packages containing 1 or 2 devices are available.
Gvoke is available as an autoinjector (Gvoke HypoPen) and prefilled syringe (Gvoke PFS) in 2 dose strengths. Adults and children aged 12 years and older as well as children aged younger than 12 years who weigh 45 kg or greater should receive 1 mg. Children aged younger than 12 years who weigh less than 45 kg should receive 0.5 mg. Both the autoinjector and prefilled syringe should be administered subcutaneously and are single use. If no response is seen in 15 minutes, another dose should be administered. Package sizes of 1 and 2 autoinjectors or prefilled syringes are available.
Both Baqsimi and Gvoke contain instructions to call for emergency assistance immediately after administration.
New drug approvals in 2019
Legislation to improve studies of medications in the pediatric patient population has increased the number of drug studies conducted in pediatric patients. However, this data often lags behind the drug’s initial complete evaluation in larger clinical trials. Here we present a review of both new drugs for pediatric patients and selected new drug approvals with high potential for pediatric use.
XENLETA (LEFAMULIN)
Xenleta is a first in-class antibiotic approved in August 2019 for the treatment of community-acquired pneumonia in patients aged older than 18 years. It works by inhibition of protein synthesis of the 50S bacterial ribosome and preventing the binding of transfer RNA for peptide transfer. In clinical studies, it has demonstrated similar rates of clinical success in patients treated with moxifloxacin with or without linezolid.9 Pediatric studies are expected to be completed in 2024.
ACCRUFER (FERRIC MALTOL)
Accrufer is an oral capsule approved in July 2019 and used for the treatment of low iron stores in adult patients.10 Pediatric studies in patients aged 1 month to younger than 10 years for pharmacokinetics and pharmacodynamics to confirm the dosing used in the efficacy and safety study are expected to be completed by January 2022.11
RECARBRIO (IMIPENEM, CILASTATIN, RELEBACTAM)
This antibiotic combination is approved to treat complicated urinary tract and complicated intra-abdominal infections in adult patients. An open-label, single-dose study evaluating pharmacokinetics, safety, and tolerability from birth to age 18 years is expected to be completed by 2021 and a randomized trial will be completed by 2024.12
ZOLGENSMA (ONASEMNOGENE ABEPARVOVEC-XIOI)
Zolgensma is a gene therapy indicated for treatment in children aged younger than 2 years with spinal muscular atrophy (SMA).13 Spinal muscular atrophy is caused by the lack of the survival motor neuron (SMN) protein due to an autosomal recessive mutation in the SMN1 gene. The lack of the SMN protein causes slow degeneration of the lower motor neurons in the spinal cord and brainstem leading to symmetric significant progressive muscle atrophy and weakness. This weakness spreads to all skeletal muscle resulting in mortality.
Onasemnogene abeparvovec-xioi is a recombinant adeno-associated virus vector-based gene therapy that delivers a normal copy of the SMN gene allowing replication of the normal protein. Unlike the alternative therapy for SMA, nusinersen, this is a one-time infusion. Significant adverse effects include acute serious liver injury, and close monitoring of liver function is recommended until 3 months after the infusion. Furthermore, there is a recommendation to administer systemic corticosteroids to all patients (prednisone 1 mg/kg/day once daily) for at least 30 days after infusion. As therapies for SMA are extremely limited, this is a life-changing option for these patients. The cost is reported to be $2.1 million per dose, making it one of the most expensive drugs on the market.14
EPINEPHRINE MULTIDOSE INHALER (PRIMATENE MIST)
Primatene Mist is an over-the-counter (OTC) multidose inhaler for asthma. Many practitioners may remember this product, which was removed from the market in 2011 due to the carbonated fluorocarbons (CFCs) in the propellant. In November 2018, Primatene Mist formulated with hydrofluoroalkanes (HFAs) as a propellant was approved for OTC use for asthma.15 Its indication is for adults and pediatric patients aged 12 years and older for the temporary relief of mild symptoms of intermittent asthma. The labeling does include a warning that it is for temporary relief of mild symptoms of intermittent asthma and that patients should see a doctor if they are feeling worse, are not better in 20 minutes, need more than 8 inhalations in 24 hours, or have more than 2 asthma attacks in 1 week.
It is important to note that this product, while it contains epinephrine, is not approved for the treatment of allergic reactions including anaphylaxis, and epinephrine autoinjectors should be used in these cases. There is little published data on inhaled epinephrine in asthma, but in a statement from the FDA, data analyzed by the agency during the drug’s review indicated no serious safety concerns if the drug was used as directed.16
Drugs with new pediatric indications
LIRAGLUTIDE
Liraglutide, originally approved in 2010 for adults with type 2 diabetes, is now approved for children aged as young as 10 years. Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue which increases glucose-dependent insulin secretion, decreases inappropriate glucagon secretion, increases beta cell growth and replication, and slows gastric emptying. Results from the Ellipse phase 3 clinical trial demonstrated an average decrease in HbA1c of 0.64 over the trial period of 26 weeks.6 The dose range is identical to adults, with a starting dose of 0.6 mg subcutaneous (SQ) daily for 1 week, then titrating to 1.2 mg if needed, and further titrating to 1.8 mg if glycemic goals are not attained on the lower doses.
This pediatric approval is the first among the newer generation of type 2 diabetes treatments. Prior to the approval of liraglutide, only insulin and metformin were approved to treat children with type 2 diabetes.
BALOXAVIR
Baloxavir was approved by the FDA in October 2018 for patients aged 12 years and older.7 It is a single-dose polymerase acidic endonuclease inhibitor indicated for the treatment of acute uncomplicated influenza. In September 2019, Roche (Basel, Switzerland) announced positive results from their MINISTONE-2 trial, the phase III study investigating single-dose baloxavir in children aged 1 to 11 years.8 Baloxavir was given as an oral suspension in the trial.
This trial randomized patients to single-dose baloxavir or usual dose oseltamivir and found “comparable” efficacy. Baloxavir was given as 2 mg/kg for patients weighing less than 20 kg or 40 mg for patients weighing 20 kg and over. The study has not yet been published and the FDA has not approved this expansion of pediatric labeling yet.
Conclusion
The past year has led to major advances in pediatric therapeutics. New dosage forms such as intranasal sprays and the trend toward subcutaneous parenteral products may be beneficial for many patients. The reintroduction of an OTC option for asthma will have to be considered by practitioners when assessing medication therapy of these patients. New antibiotics may also represent therapeutic advances in pediatric infectious diseases once pediatric data become available.
The continuing development of medications for children, better dosage forms, and pediatric dosing information all represent real progress in the quest for better medicines for children.
References:
1. Yackey K, Stukus K, Cohen D, Kline D, Zhao S, Stanley R. Off-label medication prescribing patterns in pediatrics: an update. Hosp Pediatr. 2019;9(3):186-193.
2. Czaja AS, Reiter PD, Schultz ML, Valuck RJ. Patterns of off-label prescribing in the pediatric intensive care unit and prioritizing future research. J Pediatr Pharmacol Ther. 2015;20(3):186-196.
3. Nayzilam (midazolam) [package insert]. Proximagen LLC; Plymouth, MN. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211321s000lbl.pdf. Revised May 2019. Accessed November 13, 2019.
4. Baqsimi (glucagon) [package insert]. Eli Lilly and Company; Indianapolis, IN. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf. Revised July 2019. Accessed November 13, 2019.
5. Gvoke (glucagon) [package insert]. Xeris Pharmaceuticals Inc; Chicago, IL. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212097s000lbl.pdf. Revised September 2019. Accessed November 13, 2019.
6. Tamborlane WV, Barrientos-Pérez M, Fainberg U, et al; Ellipse Trial Investigators. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019;381(7):637-646.
7. Xofluza (baloxavir marboxil) [package insert]. Genentech USA Inc; South San Francisco, CA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210854s001lbl.pdf. Revised October 2019. Accessed November 13, 2019.
8. Roche presents positive phase III study results for one-dose Xofluza in children with flu. Available at: https://www.roche.com/media/releases/medcor-2019-09-02.htm. Published September 2, 2019. Accessed November 12, 2019.
9. Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38(9):935-946.
10. Accrufer (ferric maltol) [package insert]. Shield TX (UK) Ltd; Gateshead Quays, UK. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212320Orig1s000lbl.pdf. Revised July 2019. Accessed November 13, 2019.
11. US Food and Drug Administration (FDA). FDA approves new antibiotic to treat community-acquired bacterial pneumonia. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibiotic-treat-community-acquired-bacterial-pneumonia. Published August 19, 2019. Accessed November 13, 2019.
12. US Food and Drug Administration (FDA). FDA approves new treatment for complicated urinary tract and complicated intra-abdominal infections. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-complicated-urinary-tract-and-complicated-intra-abdominalinfections Published July 17, 2019. Accessed November 13, 2019.
13. Zolgesma (onasemnogene abeparvovec-xioi) [package insert]. AveXis Inc; Bannockburn, IL. Available at: https://www.fda.gov/vaccines-blood-biologics/zolgensma. Revised May 2019. Accessed November 13, 2019.
14. Waldrop MA, Kolb SJ. Current treatment options in neurology-SMA therapeutics. Curr Treat Options Neurol. 2019;21(6):25.
15. Primatene Mist (epinephrine inhalation) [package insert]. Armstrong Pharmaceuticals Inc.; Canton, MA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205920Orig1s000lbl.pdf. Revised November 2018. Accessed November 13, 2019.
16. US Food and Drug Administration (FDA). FDA statement on approval of OTC Primatene Mist to treat mild asthma. Available from: https://www.fda.gov/news-events/press-announcements/fda-statement-approval-otc-primatene-mist-treat-mild-asthma. Published November 8, 2018. Accessed November 13, 2019.
17. US Food and Drug Administration (FDA). New pediatric labeling information database. Available at: https://www.accessdata.fda.gov/scripts/sda/sd-Navigation.cfm?sd=labelingdatabase. Updated August 31, 2019. Accessed November 13, 2019.
18. Rituxan (rituximab) [package insert]. Genentech, Inc.; South San Francisco, CA. Available at: https://www.gene.com/download/pdf/rituxan_prescribing.pdf. Revised September 2019. Accessed November 13, 2019.
19. Nucala (mepolizumab) [package insert]. GlaxoSmithKline, LLC; Philadelphia, PA. Available at: https://www.gsksource.com/pharma/content/dam/Glaxo- SmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF. Revised September 2019. Accessed November 13, 2019.
20. Enstilar (calcipotriene and betamethasone dipropionate) [package insert].: LEO Pharma Inc.; Madison, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207589s005s006s007lbl.pdf. Revised July 2019. Accessed November 13, 2019.
21. Taclonex (calcipotriene and betamethasone dipropionate) [package insert]. LEO Pharma Inc.; Madison, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022185s027lbl.pdf. Revised July 2019. Accessed November 13, 2019.
22. Botox (onabotulinumtoxinA) [package insert]. Allergan USA, Inc.; Madison, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103000s5309lbl.pdf. Revised June 2019. Accessed November 13, 2019.
23. Victoza (liraglutide) [package insert]. Novo Nordisk Inc.; Plainsboro, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022341s031lbl.pdf. Revised June 2019. Accessed November 13, 2019.
24. Aptensio XR (methylphenidate hydrochloride) [package insert]. Rhodes Pharmaceuticals LP; Coventry, RI. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205831s005lbl.pdf. Revised June 2019. Accessed November 13, 2019.
25. Lyrica (pregabalin) [package insert]. Pfizer; New York City, NY. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021446s036,022488s014lbl.pdf. Revised May 2019. Accessed Novembre 13, 2019.
26. Fragmin (dalteparin) [package insert]. Pfizer; New York City, NY. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020287s072lbl.pdf. Revised May 2019. Accessed November 13, 2019.
27. Livalo (pitavastatin) [package insert]. Kowa Company, Limited; Tokyo, Japan. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022363s015lbl.pdf. Revised May 2019. Accessed November 13, 2019.
28. Sorilux(calcipotriene) Foam. [package insert]. Mayne Pharma; Greenville, NC. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022563s006lbl.pdf. Revised May 2019. Accessed November 13, 2019.
29. Benlysta (belimumab) [package insert]. Human Genome Sciemces Inc.; Rockville, MD. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125370s064,761043s007lbl.pdf. Revised April 2019. Accessed November 13, 2019.
30. Welchol (colesevelam hydrochloride). [package insert]. Daiichi Sankyo Inc.; Basking Ridge, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210895s000lbl.pdf. Revised April 2019. Accessed November 13, 2019.
31. Avycaz (ceftazidime and avibactam) [package insert]. Allergan USA Inc.; Madison, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206494s005,s006lbl.pdf. Revised March 2019. Accessed November 13, 2019.
32. Dupixent (dupilumab) [package insert]. sanofi-aventis US LLC; Bridgewater, NJ. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s012lbl.pdf. Revised March 2019. Accessed November 13, 2019.
33. Flector (diclofenac epolamine) [package insert]. IBSA Institut Biochimique SA; Lugano, Switzerland. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021234s016s017lbl.pdf. Revised March 2019. Accessed November 13, 2019.
34. Adhansia XR (methylphenidate hydrochloride) [package insert].: Purdue Pharma LP; Stamford, C. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212038Orig1s000lbl.pdf. Revised February 2019. Accessed November 13, 2019.
35. Vfend (voriconazole) [package insert].: Pfizer Inc.; New York, NY. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021266s039,021267s050,02163s029lbl.pdf. Revised January 2019. Accessed November 13, 2019.
36. Zovirax (acyclovir sodium) [package insert]. GlaxoSmithKline; Research Triangle Park, NC. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018603s030lbl.pdf. Revised January 2019. Accessed November 20, 2019.
37. Katerzia (amlodipine) [package insert]. Silvergate Pharmaceuticals Inc; Greenwood Village, CO. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211340s000lbl.pdf. Revised July 2019. Accessed November 13, 2019.
38. Xembify (immune globulin subcutaneous, human-klhw) [package insert]. Grifols Therapeutics LLC; Research Triangle Park, NC. Available at: https://www.fda.gov/media/128601/download. Approved July 2019. Accessed November 13, 2019.
39. Dengvaxia (Dengue tetravalent vaccine, live) [package insert]. Sanofi Pasteur Inc.; Swiftwater, PA. Available at: https://www.fda.gov/media/124379/download. Approved May 2019. Accessed November 13, 2019.
40. Asceniv (immune globulin intravenous, human-sira) [package insert]. ADMA Biologics; Boca Raton, FL. Available at: https://www.fda.gov/media/122525/download. Approved May 2019. Accessed November 13, 2019.
41. Esperoct (antihemophilic factor (recombinant)) [package insert]. Novo Nordisk Inc.; Plainsboro, NJ. Available at: https://www.fda.gov/media/120351/download. Approved July 2019. Accessed November 13, 2019.