Roflumilast cream 0.05% demonstrates eczema area severity, itch improvement for pediatric atopic dermatitis
Nearly 40% of children treated with roflumilast cream 0.05% achieved a 75% improvement in EASI-75.
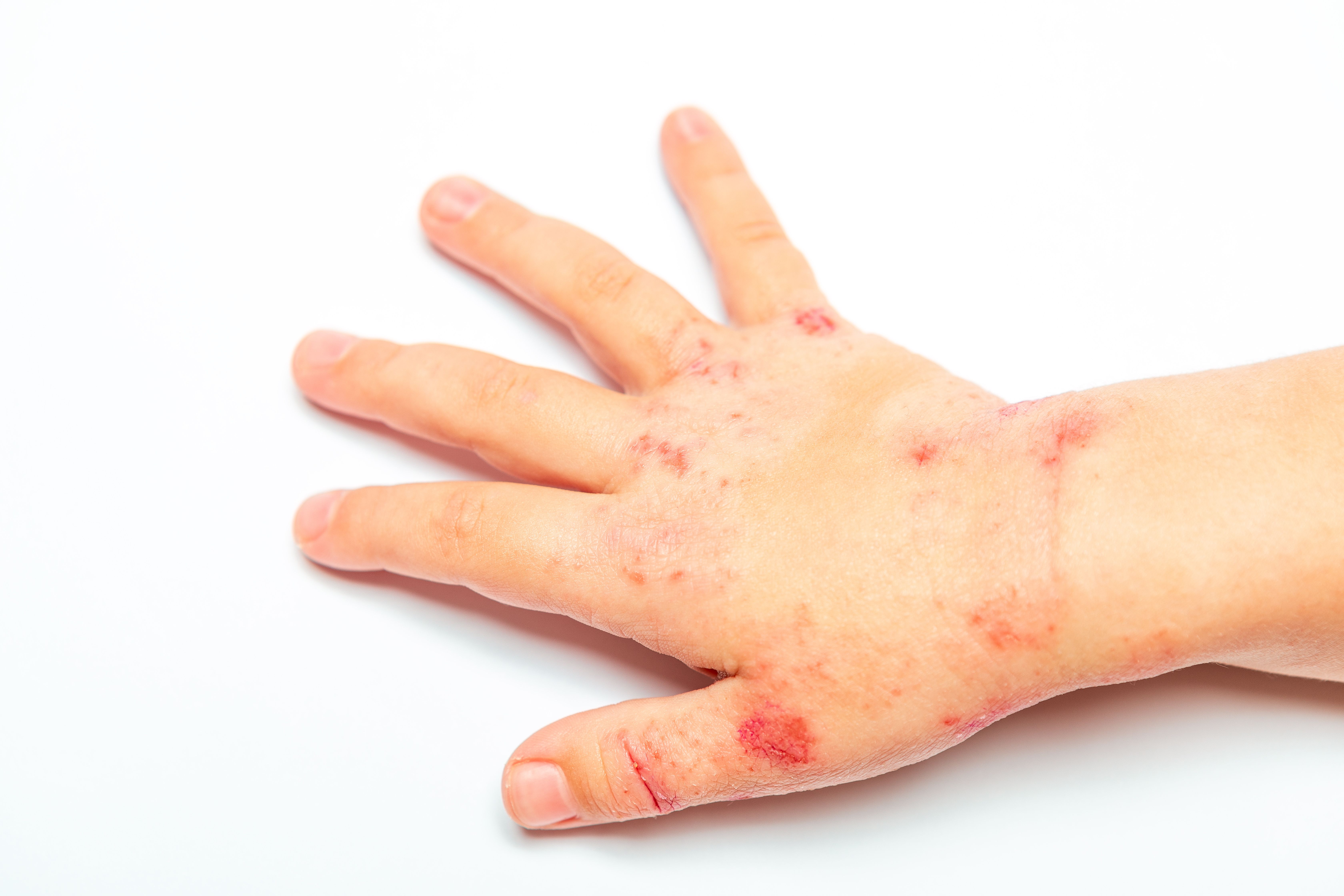
Eczema area severity, itch improvement reported for roflumilast cream 0.05% | Image Credit: © Ruslan Ivantsov - © Ruslan Ivantsov - stock.adobe.com.
Data recently published in Pediatric Dermatology detailed that roflumilast cream 0.05%(Zoryve; Arcutis Biotherapeutics) was effective in multiple key endpoints of the phase 3 INTEGUMENT-PED (NCT04845620) trial, which evaluated the safety and efficacy of the topical PDE4 inhibitor in treating atopic dermatitis (AD) among children aged 2 to 5 years.1,2
Previously reported by Contemporary Pediatrics, 25.4% of children treated with roflumilast cream 0.05% achieved validated Investigator Global Assessment-Atopic Dermatitis (vIGA-AD) success at week 4, compared to 10.7% in the vehicle-treated group (P < 0.0001), with improvements observed as early as week 1.2,3
These data, in part, led Arcutis to submit a new drug application (NDA) to the FDA for the topical cream on December 16, 2024.3
The Pediatric Dermatology publication also included that of the 437 children aged 2 to 5 years old who were treated with roflumilast 0.05%, 39.4% achieved a 75% improvement in the Eczema Area and Severity Index (EASI-75).1,2
Within the first 24 hours of roflumilast 0.05% treatment, rapid itch improvement was observed, measured by the change from baseline in daily Worst Itch Numeric Scale compared to vehicle (P ≤ .0014).2
"AD is a chronic, inflammatory skin disease affecting 1.8 million children ages 2 to 5 in the United States with burdensome symptoms that often result in impaired quality of life for both patients and their caregivers,” Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego, lead author of the Pediatric Dermatology publication, said in a statement.2
"Results from the INTEGUMENT-PED trial demonstrate that ZORYVE cream 0.05% can quickly and reliably improve the symptoms of AD, especially itch. The publication of these results and the entire clinical development program highlight that ZORYVE cream 0.05%, if approved, could fill a significant gap in the current treatment landscape for a once-daily steroid-free topical therapy that is appropriate for both the short and long-term management of AD, key concerns for young patients and their caregivers," said Eichenfield.2
WATCH: Key atopic dermatitis approvals are changing the treatment landscape
In August 2024, Contemporary Pediatrics reported on phase 3 data from the INTEGUMENT-OLE study (NCT04804605), a multicenter, open-label, extension study evaluating long-term safety of roflumilast cream 0.05% in children aged 2 to 5 years enrolled 562 individuals after completing the INTEGUMENT-PED phase 3 trial.4
In the extension study, 71.9% of participants who rolled over from the roflumilast treatment arm in INTEGUMENT-PED achieved a 75% improvement from baseline Eczema Area and Severity Index (EASI-75) after 56 weeks.4
"These results build upon the findings from the Phase 3 trial of roflumilast cream 0.05% that demonstrated rapid efficacy within the first 4 weeks of treatment, and further showed long-term durable efficacy and tolerability of investigational roflumilast cream, with continued improvement over the course of the long-term study," said Adelaide Hebert, MD, professor of dermatology and pediatrics at UTHealth Houston, and INTEGUMENT trial investigator, at the time of data publication.4
Click here for more on the INTEGUMENT-OLE study.
On July 9, 2024, the FDA approved Arcutis' roflumilast 0.15% for AD in patients aged 6 years and older, after being evaluated in a pair of identical, phase 3, parallel group, double-blind, and vehicle-controlled trials, safety and efficacy of roflumilast 0.15% was compared to vehicle and applied once daily for 4 weeks. The INTEGUMENT-1 (NCT04773587) and INTEGUMENT-2 (NCT04773600) trials featured more than 1300 adults and children aged 6 years or older with mild to moderate AD.5
References:
1. Eichenfield, LF, Serrao, R, Prajapati VH, et al. Efficacy and Safety of Once-Daily Roflumilast Cream 0.05% in Pediatric Patients Aged 2–5 Years With Mild-to-Moderate Atopic Dermatitis (INTEGUMENT-PED): A Phase 3 Randomized Controlled Trial. Pediatric Dermatology. https://doi.org/10.1111/pde.15840
2. Arcutis Announces Publication of Positive Data from INTEGUMENT-PED Trial Evaluating ZORYVE® (roflumilast) Cream 0.05% in Children 2 to 5 Years Old with Mild to Moderate Atopic Dermatitis in Pediatric Dermatology. Arcutis Biotherapeutics. Press release. February 24, 2025. Accessed February, 24 2025. https://www.globenewswire.com/news-release/2025/02/24/3031084/0/en/Arcutis-Announces-Publication-of-Positive-Data-from-INTEGUMENT-PED-Trial-Evaluating-ZORYVE-roflumilast-Cream-0-05-in-Children-2-to-5-Years-Old-with-Mild-to-Moderate-Atopic-Dermatit.html
3. Ebert, M. Roflumilast 0.05% sNDA submitted to FDA for atopic dermatitis in children 2 to 5 years. Contemporary Pediatrics. December 16, 2024. Accessed February 24, 2025. https://www.contemporarypediatrics.com/view/roflumilast-0-05-snda-submitted-to-fda-for-atopic-dermatitis-in-children-2-to-5-years
4. Fitch, J. Roflumilast cream 0.05% shows long-term efficacy in children with AD. Contemporary Pediatrics. August 28, 2025. Accessed February 24, 2025. https://www.contemporarypediatrics.com/view/roflumilast-cream-0-05-shows-long-term-efficacy-in-children-with-ad
5. Fitch, J. FDA approves roflumilast cream 0.15% for atopic dermatitis in patients aged 6 years and up. Contemporary Pediatrics. July 9, 2025. Accessed Accessed February 24, 2025. https://www.contemporarypediatrics.com/view/fda-approves-roflumilast-cream-0-15-atopic-dermatitis-patients-aged-6-years-older
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.