Shark cartilage, cat's claw, and other complementary cancer therapies
Alternative therapies seem to thrive where mainstream medicine is most challenged. The focus here is on herbs and supplements used to treat childhood cancers?why they're used, what families hope for from them, and how the evidence stacks up.
Shark cartilage, cat's claw, and other complementary cancer therapies
By Kathi J. Kemper, MD, MPH, and the Longwood Herbal Task Force
Alternative therapies seem to thrive where mainstream medicine is most challenged. The focus here is on herbs and supplements used to treat childhood cancerswhy they're used, what families hope for from them, and how the evidence stacks up.
Complementary and alternative therapies for childhood cancer, though under-tested, offer hope to families desperate for assistance. Both physicians and patients know that many medical therapies for cancer were derived from herbs: vincristine, vinblastine, and paclitaxel, for example. Medications that adversely affect the micronutrient balance of malignant cells, such as methotrexate, are also effective cancer chemotherapies.
With a broad array of herbal remedies and nutritional supplements easily available to consumers, pediatricians need to be prepared to respond to such questions as:
"We've heard that shark cartilage can kill cancer cells. Can it help my child recover from leukemia?"
"Essiac is supposed to help the immune system fight cancer. Is it safe to give along with chemotherapy? My daughter's only other medical problem is hay fever."
"My son is having problems with his liver and kidneys since he started the chemotherapy. I read on the World Wide Web that milk thistle can help with liver problems, so I've started giving him three tablets a day. Is there anything I should watch for?"
This article will familiarize you with the complementary herbal therapies and nutritional supplements that families most often use with a child who has cancer. We will review evidence on their benefits, side effects, and potential interactions with mainstream therapies.1 We do not suggest that herbs, vitamins, or supplements be used in place of conventional care; we do suggest that some complementary remedies, in conjunction with mainstream treatments, will do little or no harm. Although peer-reviewed scientific studies evaluating the use of these products with children are in short supply, their absence does not necessarily mean that an alternative therapy is ineffective or dangerous. Many herbs and dietary supplements are traditionally avoided during pregnancy and early childhood not because toxicity has been proved but because of the strong caution needed in these sensitive developmental periods.
Prevalence of CAM use
In 1997, approximately 40% of American adults used some kind of complementary or alternative medicine (CAM).2 While percentages tend to be lower among general pediatric patients, as many as 50% to 70% of children with cancer seek CAM care.3 Rates are particularly high among children who have had relapses.4
Many pediatricians are concerned about the potential harm from such therapies.5 On the other hand, some ethicists argue that for patients with terminal illness, it is not unethical to support alternative treatments as long as they are neither toxic nor costly.6
Herbs and dietary supplements are not as tightly regulated as pharmaceutical products, and some of them have substantial potential for toxicity and interactions with mainstream treatments. Manufacturers are not required to prove safety or efficacy before marketing their products, even those meant specifically for children. Although manufacturers cannot legally claim that these products prevent, treat, or cure a specific disease, they can make claims about function that consumers may interpret as being helpful for treating cancer, such as "supports healthy immune function."
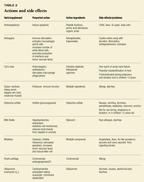
Herbs and supplements are typically used for three reasons: to kill cancer cells, to promote overall well-being and enhance the immune response, and to treat symptoms and side effects. Table 1 groups the products discussed into these categories, and Table 2 summarizes their action, active ingredients, and side effects.
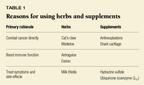
Combating cancer
Herbs and dietary supplements used primarily for their direct anticancer effects include cat's claw, mistletoe, antineoplastons, and shark cartilage. Some of these also have immune-stimulating properties.
Cat's claw or una de gato. The bark of the root from South American cat's claw (Uncaria tomentosa) is used both to kill cancer cells and to support the immune system. People who live in the rain forests of Peru have used cat's claw for 2,000 years to prevent disease and pregnancy and to treat inflammatory diseases and cancer. Modern herbalists recommend cat's claw as an immune stimulant, an anti-inflammatory, and an antimicrobial agent. Some essiac tea mixtures (discussed below) now contain cat's claw in addition to the traditional ingredients. A European product, Krallendorn tea or capsules, also contains cat's claw.
In vitro, cat's claw extracts, including triterpenoid saponins, demonstrate antimutagenic and antitumor effects. The herb's oxindole alkaloids, notably uncarine F, are also active against several lines of leukemia cells. Cat's claw does appear to be effective in treating feline leukemia virus, and case reports on the effectiveness of cat's claw given to Peruvian children with leukemia are promising. However, no peer-reviewed, controlled trials have been published evaluating the effectiveness of cat's claw, either alone or in combination with conventional therapies, for leukemia or other forms of cancer.
In addition to its use as a direct anticancer agent, cat's claw is used to stimulate the immune system. Its oxindole alkaloids, particularly isopterodine and pterodine, stimulate phagocytosis in vitro; mice given root bark extract had a 30% to 40% increase in macrophage activity. Similar data in oncology patients have not been reported.
The typical recipe for cat's claw root tea is 20 to 30 g, finely chopped and boiled in one quart of water for three hours until the volume is reduced to about one third. The tea is cooled to room temperature and sipped three times a day. In capsule form, the usual adult dosage is 350 to 500 mg once or twice daily. Taken as a tincture, the dosage is 1 to 2 mL up to twice daily.
Cat's claw is traditionally contraindicated during pregnancy; in children under 3 years of age; in patients taking insulin or thymus extracts; in recent vaccine recipients; and in those who have recently received immune globulin or sera. Physiologic data supporting these contraindications are lacking. However, one case report described acute renal failure in an adult who was taking cat's claw to treat systemic lupus erythematosus. Extra vigilance toward renal function may therefore be warranted in patients who use cat's claw as an adjunctive therapy.
The potential for misidentification or mislabeling of commercial cat's claw products is a concern. Some manufacturers substitute Acacia gregii, a plant that grows along the Texas-Mexico border, for South American cat's claw in products sold in America. A gregii contains a potentially poisonous cyanide-based chemical compound. In addition, 12 species in Peru besides cat's claw are called una de gato, and throughout the world there are 34 species of Uncaria that have medicinal properties. Asian species of Uncaria are used as sedatives and antihypertensives. Given this variability and the weak quality control of herbal products, patients who decide to use cat's claw remedies should be very closely monitored.
Mistletoe. Recognizing its potential toxicity, mothers and grandmothers have warned children for years not to eat mistletoe (Viscum album Loranthaceae). But the chemical constituents that make mistletoe toxic may also contribute to its effectiveness against cancer. Like cat's claw, mistletoe is used both to kill cancer cells and to enhance the immune system. Its use as a chemotherapeutic agent was first suggested in 1916 by the anthroposophist Rudolf Steiner. It is now one of the most widely used herbal treatments for cancer in Europe, particularly Germany and Switzerland, where it is sold under the brand names Iscador (Switzerland), Eurixor (Germany), Helixor (Germany), and Isorel (Austria).
Mistletoe's active ingredients vary somewhat by subspecies, growing conditions, and commercial preparation, but generally include viscotoxins and lectins. The amino acid arginine, both a tumor inhibitor and an immune stimulant in animals, is also present in unusually large amounts (16.8%) in mistletoe juice. Viscotoxins directly damage cell membranes. Lectins are glycoproteins that bind various sugars. They stimulate the immune system and inhibit protein synthesis in cancer cells via enzymatic inhibition of ribosomes. In vitro studies suggest that lectins are cytostatic against tumor cells and may induce apoptosis (programmed cell death). In rodents, mistletoe extracts can prevent tumor metastases.
More than 40 studies document the use of mistletoe as a treatment for cancer in humans, primarily for solid tumors such as breast, colorectal, gastric, cervical, and renal tumors. Few of these studies were randomized trials, however, and reports on effectiveness are mixed. No published randomized controlled trials have evaluated the safety and effectiveness of mistletoe in treating pediatric oncology patients. Manufacturers do not promote it for treating leukemia, the most common form of childhood cancer.
Mistletoe is also used to enhance the immune response. It induces tumor necrosis factor (TNF) production by macrophages. In vitro and in animal models, mistletoe increases natural killer cell activity, increases the production of interleukins, and activates macrophages. In animal studies, it increases the weight of the spleen and thymus, suggesting increased numbers of active immune cells. No studies have specifically evaluated mistletoe's effectiveness in stimulating the immune system in pediatric patients with cancer.
In Europe, mistletoe extracts are typically administered by subcutaneous injection three to seven days per week. The preparation is sometimes injected directly into solid tumors. Side effects include anaphylactic allergic reactions. Many patients become febrile and experience flu-like symptoms, including nausea and abdominal pain. As with any injection, the site may become inflamed. Ingestion by young children may lead to seizures and coma. Because of its tyramine content, mistletoe is contraindicated in patients taking any type of monoamine oxidase (MAO) inhibitor.
Antineoplastons. At Baylor College of Medicine in the 1970s, chemist Stanislaw Burzynsky developed the theory that certain peptide fractions and other molecules could trigger apoptosis in neoplastic cells. He called these peptide fractions, amino acid derivatives, and organic acids "antineoplastons." Burzynsky theorized that antineoplastons are the backbone of an innate defense system against cancer that parallels the immune system. He postulated that antineoplastons reprogram defective cells rather than engulfing or destroying them. Antineoplastons are hypothesized to be species specific, making animal testing of human antineoplastons virtually meaningless. This theory has generated heated controversy, with proponents labeling antineoplastons "experimental" and opponents calling them "unproven"a semantic difference with powerful political, scientific, and clinical consequences.
In vitro, antineoplastons profoundly inhibit oncogene expression, limit the proliferation of malignant cells, and induce terminal differentiation (reversion toward normal cell structure). Antineoplastons proved ineffective in treating P388 mouse leukemia, the standard National Cancer Institute (NCI) screening test for antitumor drugs in the early 1980s. Case series from investigators in Japan and elsewhere, however, reported promising results.
In 1991, the NCI reviewed seven of Burzynsky's best cases and concluded that antitumor effects were achieved. The Food and Drug Administration (FDA) has approved phase 2 studies to test the efficacy of antineoplaston treatment in breast cancer and several types of brain tumor; pediatric patients are included in the testing. As yet, no randomized controlled trials of the effectiveness of antineoplastons in treating pediatric oncology patients have been published.
Antineoplastons are typically administered parenterally but are also available in oral preparations. The average treatment (obtainable in the United States only as part of experimental protocols) lasts four to 12 months and costs $36,000 to $60,000. Side effects are uncommon and tend to be mildchills, fever, stomach upset, and mild rashes. One unusual side effect is a strong body odor similar to urine.
Shark cartilage. When the National Institutes of Health (NIH) National Center for Complementary and Alternative Medicine (NCCAM) opened in the early 1990s, it received a flood of telephone inquires about cancer remedies. The most frequent question concerned the efficacy of shark cartilage in curing cancer. In 1994, an estimated 50,000 Americans were using shark cartilage, at costs averaging $7,000 per person per year. Since the original reports and speculations regarding Pacific shark cartilage were publicized, other types of cartilage (such as bovine) have also been promoted.
Substantial controversy exists regarding the mechanism of action for cartilage supplements. In the 1970s and 1980s, in vitro and animal studies identified antiangiogenesis factors in shark cartilage. However, the antiangiogenesis peptides in shark cartilage appear to be destroyed during the digestive process, limiting the usefulness of oral preparations. Some suggest that mucopolysaccharides in cartilage block mitosis in tumor cells. In 1998, a large manufacturer of shark cartilage announced the isolation of anti-matrix metalloprotein inhibitors, which reduce the ability of cancer cells to metastasize. Other researchers believe that shark cartilage's most important action is to stimulate macrophages.
The majority of scientific reports of shark cartilage use in humans have been case reports and case series, many from outside the United States. Although several case series suggest promising results in adults with various solid tumors (breast, prostate, lung, colon, renal, and others), the lack of control or comparison groups makes it difficult to assess the effectiveness of cartilage compared with other therapies used by these patients.
The FDA has approved an Investigational New Drug application for a shark cartilage product, Benefin, to evaluate its effectiveness in treating prostate cancer and Kaposi's sarcoma. Currently, there are no published randomized controlled trials evaluating the effectiveness of shark cartilage in treating children with cancer.
The usual adult daily dosage is 90 to 110 g orally (1 to 1.25 g/kg). Dogfish shark cartilage, Cartilade, is available in 200-, 500-, and 740-mg gelatin capsules, requiring dozens to hundreds of capsules daily to reach recommended doses. Patients who use recommended doses are likely to incur substantial out-of-pocket costs. Allergic reactions may occur. In a phase 1 study of 47 patients taking cartilage products as adjunctive therapy for advanced cancers, four had to stop treatment because of significant toxicity. Safety and toxicity in children have not been determined.
Boosting immune function
Astragalus root and the multiple-ingredient essiac formula are herbal remedies reputed to enhance immune system function.
Astragalus. For thousands of years, astragalus (Astragalus membranaceus) root has been used in China as a liver tonic, antihypertensive, mild anticoagulant, and immune stimulant to combat viral infections. In traditional Chinese medicine it is typically included in a complex herbal mixture to treat coronary artery disease and congestive heart failure; other mixtures, such as Fu-zheng, are used to treat infections and cancers. Astragalus is rarely used alone.
In vitro, astragalus extracts improve T-lymphocyte function, stimulate interferon production, and increase activation of macrophages and B cells. Chinese in vitro studies of astragalus in numerous types of cancer demonstrate restored T-cell function as well as enhanced TNF production and recovery of macrophage function.
Astragalus extracts administered to mice potentiated the antitumor effects of interleukin-2, reducing the required cytotoxic dose. Mice who were immunosuppressed by chemotherapy or radiation demonstrated enhanced B-cell activity, heightened antibody response to foreign antigens, and enhanced lymphokine-activated killer cells and macrophage activity following treatment with astragalus.
Leukopenic adults treated with 15 g of astragalus twice daily for four weeks exhibited significantly increased numbers of peripheral white blood cells. In several Chinese randomized trials of adults with cervical cancer, hepatomas, lung cancer, and breast cancer who had been treated with radiotherapy, patients who received an astragalus-containing herbal mixture had improved survival compared with those treated with radiotherapy alone. Notably, astragalus was not given as an isolated herb, but as part of a complex herbal compound. Astragalus's effects in children with cancer have not been assessed in randomized controlled trials.
Concentrations of the herb's active polysaccharides, astragolosides and trigonosides, differ markedly depending on growing conditions. The usual adult dosage is 1 to 4 g taken three times daily as a tea. If taken as a powdered root extract, one to three 250- to 500-mg capsules are taken three times per day. As a tincture, the dosage is 3 to 6 mL by mouth three times daily. Appropriate pediatric dosages are not known.
No adverse effects have been noted with up to three months of daily use in adults. Traditional herbalists recommend that astragalus not be used for more than three consecutive weeks and that use be discontinued during acute febrile illnesses. Due to potential herb-drug interactions, patients should be cautious when taking diuretic, fibrinolytic, antihypertensive, and inotropic medications. The safety of astragalus during pregnancy, lactation, and early childhood is unknown.
Essiac. One of the best known and most widely used herbal cancer remedies is essiac. It was first extensively used and promoted by an Ontario nurse, Rene Caisse (essiac reversed). One of her patients reported being healed of fulminant breast cancer by an herbal formula that an Ojibwa medicine man gave her in 1922. The original formulawhich Caisse kept secret but used to treat hundreds of Canadian cancer patientsincluded a tea made from four dried herbs: burdock root, the inner bark of the slippery elm tree, sheep sorrel, and turkey (medicinal) rhubarb root. Newer formulations may also contain blessed thistle, cat's claw, red clover, kelp, and watercress.
The effectiveness of the original herbal formula has been hotly debated for more than 50 years. Despite numerous inspiring testimonials and case reports promoting the nearly miraculous benefits of essiac, the preparation has undergone little scientific study. There are no published prospective controlled trials on the use of essiac to treat any form of pediatric cancer. In 1977, the Canadian government sponsored a five-year clinical trial on the treatment of advanced cancer in adults; essiac was neither curative nor palliative.
The usual adult dosage of essiac is as a tea taken twice daily, either two hours before or two hours after a meal. As a tincture, the dosage is 1 oz one to three times daily. Contamination of ingredients may occur. Allergic reactions are possible. Reported side effects of essiac include diarrhea, nausea, vomiting, headache, and increased urination. Essiac should not be used by pregnant or nursing women, children younger than 2 years, those with known hypersensitivity to any of its ingredients, or patients with a history of renal stones.
While the data on essiac's component herbs are variable, they are generally more extensive than published data on the compound itself.
- Burdock root (Arctium lappa) is eaten as a vegetable (gobo) in Japan. The German mystic and healer Hildegard of Bingen used it as a cancer treatment, and it is an ingredient in the Hoxsey herbal cancer tonic. One of burdock's active ingredients, arctigenin, appears to have antimutagenic effects and inhibits tumor growth in vitro; one study reported necrosis of solid tumors in mice treated with burdock. Its effectiveness in treating pediatric cancer has not been evaluated.
- Burdock root may be confused with the similar-looking roots of Atropa belladonna during the harvesting process. Since much medicinal burdock is grown in Bulgaria, Yugoslavia, Poland, and Hungary, where quality control is limited, consumers must be vigilant for atropine-type poisoning from contaminated lots. Herbalists traditionally recommend avoiding burdock during pregnancy and lactation, and in children younger than 2 years.
- Slippery elm bark (Ulmus flava)is a well-known demulcent that soothes inflamed mucous membranes. It is widely available in teas and lozenges for the symptomatic treatment of sore throats and upper respiratory infections. It has no known effect on the immune system or on cancer cells.
- Sheep sorrel (Rumex acetosella) is a traditional source of "spring greens." The plant contains 7% to 15% tannins, powerful astringents that may cause upset stomach and renal and hepatic damage; 0.3% oxalic acid, which may lead to calcium oxalate renal stones in persons with a history of kidney stones; vitamin C; and small amounts of anthraquinone glycosides, which may cause diarrhea. No studies have evaluated its effectiveness in treating pediatric cancer. Sorrel is better known as a cause of hay fever. Allergic reactions are common.
- Rhubarb root (Rheum palmatum) and its relative, R officinale, have been extensively used and investigated as medicinal agents in Asia. Rhubarb is traditionally used as a diuretic and an ulcer remedy. Recently, it has undergone investigation as an antimicrobial agent and as a treatment for chronic renal failure. In small amounts, it helps treat diarrhea, but in larger doses, due to its concentration of anthraquinone compounds, it acts as a laxative. Rhubarb root also contains tannins and oxalic acid. It is not recommended for use during pregnancy or lactation or in children under 2 years old. It should be avoided in patients with intestinal obstruction and in those with a history of renal stones. Its interaction with standard chemotherapeutic agents has not been studied, but there are no reports of adverse interactions.
Treating symptoms and side effects
A well-known herb and two naturally occurring substances may help alleviate some cancer symptoms and provide protection against treatment-induced toxicity.
Milk thistle.The seeds of the milk thistle plant (Silybum marianum) have been used in Europe for hundreds of years to treat hepatobiliary problems. The main active ingredient, silymarin, contains three flavonlignans: silybin (silibinin), silidianin, and silychristin.
In rats and mice, silymarin has proven protective against a number of known hepatotoxins, including carbon tetrachloride, alcohol, acetaminophen, and viruses. Silymarin appears to act as an antioxidant and may increase protein synthesis by stimulating ribosomal RNA polymerase and DNA synthesis. It also alters hepatocyte membrane permeability, decreasing permeability to toxins. In humans, milk thistle extracts have been effective in treating alcoholic hepatitis and cirrhosis and as an antidote for Amanita mushroom poisoning. Current studies are evaluating its efficacy in treating viral hepatitis, including hepatitis C.
Rats given milk thistle before exposure to cisplatin experienced less nephrotoxicity (as measured by blood urea nitrogen, creatinine clearance, and histology) than untreated rats. Milk thistle has not been systematically evaluated as an adjunctive therapy for pediatric oncology patients with renal or hepatic dysfunction.
Silymarin is rapidly absorbed after oral administration, reaching peak concentrations in two hours, with an elimination half-life of six hours. It is poorly absorbed in water, so teas are an ineffective route of administration. In Europe, silymarin is given parenterally to treat acute hepatotoxicity. Standardized oral tablets contain 70% silymarin, that is, a standardized 200-mg tablet contains 140 mg of silymarin. The typical adult dosage for milk thistle is 200 to 420 mg per day (for Amanita mushroom poisoning, 20 to 50 mg/kg per day). In 1998 the cost for oral treatment was about 50 cents to $2 daily. Toxicity with milk thistle is unusual. Animal studies have shown silymarin to be nontoxic at doses of 2,500 to 5,000 mg/kg of body weight. Allergic and mild laxative reactions have been reported but are rare.
Hydrazine sulfate. A monoamine oxidase (MAO) inhibitor, hydrazine sulfate is used to treat cancer-associated cachexia. It was developed by Dr. Joseph Gold based on the theory that the higher energy metabolism in tumor cells demands higher rates of gluconeogenesis to convert lactic acid to glucose. Hydrazine sulfate interrupts gluconeogenesis by blocking phosphoenolpyruvate carboxykinase, reducing the energy available for tumor growth.
Case reports and case series of seriously ill patients given hydrazine sulfate offer promising testimonials about its ability to reverse cachexia and even slow or reverse tumor progression and prolong survival. At least eight randomized controlled trials have studied the use of hydrazine sulfate in the treatment of adult oncology patients, five of which involved patients with lung cancer. Nearly all patients had advanced or metastatic disease. Four studies found that patients had modest improvements in weight loss, carbohydrate metabolism, and serum albumin (a marker of nutritional status). However, three trials showed that patients failed to improve, and one demonstrated poorer survival and quality of life among hydrazine-treated patients. None have specifically addressed the effectiveness of hydrazine sulfate in treating pediatric oncology patients.
The usual adult dose of hydrazine sulfate (available commercially as Sehydrin) is 60 mg by mouth four times daily for several days, tapering to two or three times daily for one to two months. Side effects of hydrazine sulfate include nausea, vomiting, dizziness, paresthesias, weakness, polyneuritis, insomnia, and pruritus. Long-term use may be hepatotoxic. Patients taking barbiturates, alcohol and other central nervous system depressants, and foods high in tyramine should avoid hydrazine. Hydrazine should not be used during pregnancy or lactation. There are no data on the safety of its use in children.
Coenzyme Q10 or ubiquinone. Known as coenzyme Q10, CoQ10, and ubiquinone (2,3-dimethoxy-5-methylbenzoquinone), this dietary supplement is not an herb but a naturally occurring ubiquinone structure found in most aerobic organisms; it can also be chemically synthesized. Humans synthesize ubiquinone from a cascade originating with tyrosine; it is a fat-soluble quinone with structural similarities to vitamin K. Since the 1950s, it has been used as an adjunctive therapy for various cardiovascular diseases, including congestive heart failure, hypertension, cardiomyopathy, angina, and doxorubicin-induced cardiotoxicity.
Ubiquinone has four primary physiologic roles: (1) in the mitochondrial production of adenosine triphosphate, (2) in extramitochondrial redox activity, (3) as an antioxidant scavenger of lipid radicals, and (4) as a contributor to membrane stability and fluidity. The last two activities are believed to account for its cardiac benefits.
Treating animals with ubiquinone before experimentally induced ischemia results in better cardiac contractility compared with control animals. Rats with congestive heart failure demonstrated improved contractility when treated with CoQ10. Pretreatment with CoQ10 was also cardioprotective in several studies of rats given anthracyclines, such as doxorubicin.
In randomized controlled trials, adults with angina pectoris who were given CoQ10 (50 to 150 mg three times daily for four weeks) had significantly improved exercise tolerance and fewer electrocardiographic changes compared with controls. Adults with severe congestive heart failure showed significant improvements in stroke volume, ejection fraction, cardiac index, rate of hospitalizations, and survival after taking CoQ10 daily for 3 to 12 months. In another study, treating adults with CoQ10 for three to five days before chemotherapy with doxorubicin protected against decreases in stroke volume, ejection fraction, and cardiac index.
We found no published studies on CoQ10's effectiveness in treating children with congestive heart failure. However, given the consistently beneficial results in animal and adult studies, CoQ10 appears highly promising as an adjunctive therapy for children undergoing chemotherapy with cardiotoxic medications, particularly those whose chemotherapy may be limited by the risk of severe cardiac side effects. Use for these children must still be regarded as experimental, however. Because CoQ10 is widely available without a prescription, physicians should inquire whether families have already started or have considered using this therapy. Some families may want to consider collaborating with national and regional trials to evaluate CoQ10's therapeutic effectiveness.
The typical adult dosage of CoQ10 is 100 mg daily. The supplement can be purchased in most retail pharmacies in concentrations ranging from 30 to 120 mg per capsule. Nausea, anorexia, abdominal pain, and diarrhea are the most common side effects. The reported incidence of all gastrointestinal effects is less than 5%. No neurologic, cardiovascular, renal, hepatic, teratogenic, or mutagenic effects have been noted. Doses as high as 45 mg/kg per day have been used without notable toxicity. There have been no reports of hypersensitivity to CoQ10. No adverse drug interactions have been reported.
In essence
Families that have a child suffering from cancer are desperate for hopes of nontoxic cures. Numerous herbal remedies and dietary supplements are marketed to treat cancer, boost the immune system, and reduce the side effects of standard treatments. Although some rationales are compelling and case series inspiring, few of these remedies have been adequately tested in children. Consumers should be aware of the caveats listed in Table 3. Herbal products and food supplements are not federally regulated or held to the same standards as medications, and manufacturers need not prove a product is safe or effective before marketing to children.

Pediatricians can consult the publications and Web sites in "Resources for pediatricians" for additional information. They should routinely ask families about the complementary therapies they have tried or considered for their child; inquire about perceived benefits, costs, and side effects; and offer nonjudgmental information to assist in their decision making. Pediatric cancer research groups should place a high priority on rigorously evaluating the safety and efficacy of the supplements and herbs often used by children with cancer.

DR. KEMPER is Director, Center for Holistic Pediatric Education and Research, Children's Hospital, Boston, and Associate Professor, Harvard Medical School, Boston, MA.
LONGWOOD HERBAL TASK FORCE members include volunteer faculty, staff, and students from the Massachusetts College of Pharmacy, Children's Hospital, and the Dana Farber Cancer Institute, Boston.
REFERENCES
1. Extensive references on each supplement are available at the Longwood Herbal Task Force Web site, http://www.mcp.edu/herbal/default.htm. Another Web site that covers herbs and other common natural remedies for cancer is http://www.sph.uth.tmc.edu/utcam.
2. Eisenberg D, Davis R, Ettner S, et al: Trends in alternative medicine use in the United States, 19901997. JAMA 1998;280:1569
3. Friedman T, Slayton W, Allen L, et al: Use of alternative therapies for children with cancer. Pediatrics 1997;100:1
4. Grootenhuis MA, deGraaf-Nijkerk JH, van der Wel M: Use of alternative treatment in pediatric oncology. Cancer Nurs 1998;21:282
5. Coppes MJ, Anderson RA, Egeler RM, et al: Alternative therapies for the treatment of childhood cancer (Letter). N Engl J Med 1998:339(12):846
6. Jackson J: Unproven treatment in childhood oncologyhow far should paediatricians cooperate? (Commentary) J Med Ethics 1994;20:77
Kathi Kemper. Shark cartilage, cat's claw, and other complementary cancer therapies. Contemporary Pediatrics 1999;11:102.
Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.
Meet the Board: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI
May 20th 2022Contemporary Pediatrics sat down with one of our newest editorial advisory board members: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI to discuss what led to her career in medicine and what she thinks the future holds for pediatrics.
Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.
Meet the Board: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI
May 20th 2022Contemporary Pediatrics sat down with one of our newest editorial advisory board members: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI to discuss what led to her career in medicine and what she thinks the future holds for pediatrics.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.