Three technologies for taming otitis media
Every day pediatricians must diagnose middle ear disease and decide whether to prescribe antibiotics. To help meet this challenge, consider using the technologies described here--tympanometry, spectral gradient acoustic reflectometry, and distortion product otoacoustic emissions audiometry.
Three technologies for taming otitis media
By Jerome T. Combs, MD, and Andrew J. Schuman, MD
Every day pediatricians must diagnose middle ear diseaseand decide whether to prescribe antibiotics. To help meet this challenge,consider using the technologies described here--tympanometry, spectral gradientacoustic reflectometry, and distortion product otoacoustic emissions audiometry.
Otitis media accounts for more than 42% of antibiotics prescribed tochildren. In view of the rise in infections caused by antibiotic-resistantbacteria, the Centers for Disease Control and Prevention and the AmericanAcademy of Pediatrics have advised physicians to prescribe antibiotics moreprudently.1,2 In addition, some pediatricians question whetherall of the 2 million surgical procedures each year to insert pressure-equalizing(PE) tubes--the most common surgical procedure requiring anesthesia in thiscountry--are truly necessary.1
To reduce prescriptions for antibiotics and inappropriate referrals fortubes, pediatricians need to improve their ability to diagnose and monitormiddle ear disease. The three office technologies we discuss here can helpyou achieve these goals. The tympanometer is a time-tested instrument forassessing and monitoring middle ear effusion (MEE), and two newer technologies,spectral gradient acoustic reflectometry (SGAR) and distortion product otoacousticemissions (DPOAE) audiometry, are also available (Table 1). Before describinghow the technologies work and the way to use them to improve managementof otitis media, we will review the diagnostic and treatment challengesassociated with this condition.

Making judgments
Pediatric training programs continue to emphasize the importance of performingpneumatic otoscopy during examination of the child with an earache, andit has remained the mainstay of diagnosis for decades. Many pediatriciansdo not routinely use pneumatic otoscopy, however, and others perform itincorrectly.3 Ideally, pneumatic otoscopy permits the pediatricianto assess the appearance of the tympanic membrane (translucent, cloudy,red), its position (normal, retracted, bulging) its mobility, and the presenceof fluid in the middle ear space. In real life, though, an uncooperativechild can lead to misdiagnosis, as can an inadequate pressure seal betweenthe speculum and ear canal, which makes it difficult to assess mobility.The tympanic membrane also may be obscured by cerumen, and the infectedtympanic membrane of a crying child can be misinterpreted as inflammation.Although observers sometimes criticize pediatricians for overdiagnosingacute otitis media, these problems make it just as easy to miss a case,particularly when the child is examined early in the course of the disease.
Once the correct diagnosis has been made, to use or not to use antibioticsbecomes the central question. During the past decade, more and more of thebacterial pathogens isolated from children with acute otitis media (AOM)have proved to be penicillin resistant, raising serious questions aboutthe role of antibiotics in the treatment of AOM and otitis media with effusion(OME). As Table 2 shows, current recommendations emphasize distinguishingAOM, for which antibiotics may be appropriate, from OME, for which antibioticsshould not be used. AOM is characterized by inflammation of the tympanicmembrane and pus in the middle ear space, often accompanied by systemicsigns of illness including ear pain and fever. In OME, only fluid is inthe middle ear space.

In some Scandinavian and European countries, antibiotics are prescribedfor AOM in children over 2 years of age only if symptoms and signs persistfor more than a few days.4 Physicians in the US, however, continueto favor acute antibiotic treatment of AOM in all age groups. Such therapyhas been shown to improve cure rates by approximately 14% and to reducethe incidence of the suppurative complications of AOM such as mastoiditis.5Pediatricians now can elect to use a traditional 10-day course of antibioticsor consider abbreviated treatment regimens, including a five-day courseof antibiotics or a single intramuscular injection of ceftriaxone in childrenolder than 2 years.6 Five years ago, the Agency for HealthcarePolicy and Research (AHPR) recommended using antibiotics in the initialtreatment of OME in children between 1 and 3 years of age. Infectious diseasespecialists now advise against this practice, but agree with AHPR that antibioticscan be considered if OME persists beyond three months and is associatedwith a bilateral threshold hearing level of 20 decibels (dB) or higher.Tube placement can be considered if the effusion persists beyond four tosix months and is associated with a bilateral hearing deficiency.7
The three technologies described here can be used to diagnose AOM, toidentify circumstances in which antibiotics can be postponed or avoidedaltogether, and to monitor a patient's progress with or without antibiotictherapy. They can also help diagnose OME associated with upper respiratoryinfections or allergies, and residual OME that follows AOM.
Tympanometry
Tympanometry, coupled with otoscopy, is the high-tech equivalent of pneumaticotoscopy. While experts advocate pneumatic otoscopy, they invariably recommendturning to tympanometry when the observer is confused or uncertain. Pneumaticotoscopy uses light energy to determine movement of the tympanic membrane;tympanometry uses sound energy to determine this movement as well as pressurein the middle ear. Pneumatic otoscopy is subject to observer bias, whiletympanometry is completely objective and produces a permanent "snapshot"of the tympanic membrane at the time of the examination.
Many fine tympanometry devices are available. They all operate by thesame physical principle, they all provide hard copy, and they all cost from$2,500 to $3,000. Some offer pure tone screening or threshold audiometryfor a modest additional price. All but one have a probe that is attachedto a sizable desk-top device that is plugged into the wall. The exceptionis the MicroTymp 2 made by Welch Allyn. Its instrument is a portable deviceabout the size of a large otoscope and prints out hard copy in about fiveseconds when placed in a small printer/charger unit. A recent validationstudy found that this instrument had a sensitivity of 71% and specificityof 93% in documenting middle ear effusion. Measurements took an averageof two minutes to perform.8
All tympanometric instruments measure the change in sound energy thatoccurs in a closed chamberas its volume changes. A transducer generatesa pure tone of 226 Hz with an intensity of 85 dB. A pressure seal is madeat the opening of the patient's ear canal and a miniature air pump pressurizesthe ear canal to 200 dekapascals (daPa, a unit of pressure equal to 1.02mm of water). The instrument instantaneously calculates the volume of theear and then sucks out air, reducing the pressure to 400 daPa in abouttwo seconds. The movement (compliance) of the tympanic membrane is thendisplayed graphically.
If the middle ear space is completely filled with fluid, the tympanicmembrane does not move at all, and nothing appears in the window of thegraph the instrument produces. A normal tympanic membrane with no effusionor abnormal pressure behind it will show a nice sharp narrow tracing inthe window--in the lower window pane for infants and children and the upperpane for older children and adults (Figure 1). In explaining a normal tracingto a parent, it may be helpful to describe it as an tree seen through awindow.

Physicians tend to think the middle ear space is either completely fullor empty. When we see a tympanic membrane that has an air or fluid levelor bubbles behind it, we are reminded that some effusions are partial. InAOM, however, the tympanic membrane is generally opaque, and only pneumaticotoscopy or tympanometry makes it possible to discern a partial effusion.Figure 2 illustrates the tympanograms for a variety of clinical conditions.9

Because a normal curve in the window cannot be obtained in error, thistracing is always reassuring. If the tympanic membrane is red and swollenbecause of fever, for example, the normal tracing it elicits suggests thatantibiotics are inappropriate. Tympanometry also makes it unnecessary toremove cerumen that is obstructing visualization of the entire tympanicmembrane; the device's tracings will be valid and reproducible since soundand air pressure can go around corners where the eye can't reach.
Impacted wax or bad technique can produce a flat tracing, but the volumeof the external ear will be shown as too small. An open PE tube can producea flat curve with a large ear volume.
Spectral gradient acoustic reflectometry
Modern SGAR has evolved from the acoustic otoscope, which was introducedinto the pediatric office 14 years ago to supplement information providedby routine tympanometry and pneumatic otoscopy. The acoustic otoscope, whichwas developed by a pediatrician and sonar engineer, was a hand-held devicethat analyzed sound waves bounced off the tympanic membrane to determinethe presence of a middle ear effusion. The instrument emitted a spectrumof sound, delivered in short pulses, from 1,619 to 4,724 Hz (1.6 to 4.7kHz) with an intensity of 80 dB. A microphone detected the sum of emittedand reflected sound and displayed a graph of the reflectivity in arbitraryunits from 0 to 9 on the graph's vertical axis.
The amount of sound energy bouncing back from the tympanic membrane rosewith the reflectivity and the likelihood that an effusion was present. Unliketympanometric devices, the first-generation acoustic reflectometer did notrequire a seal between the instrument and the ear canal. Although the technologywas based on valid engineering and physiologic principles, it was not widelyaccepted, in part because the instrument was expensive and subject to technique-relatederrors. If the device was not positioned correctly, sound energy could escapearound the sensor, producing falsely low reflectivity readings. Studiesshowed that the sensitivity of the device in predicting MEE varied from54% to 94%, and its specificity ranged from 62% to 84%.10
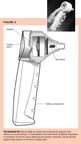
In 1997, MDI Instruments began to market a second-generation acousticreflectometer called the EarCheck Pro; it is based not on the intensityof the reflected sound, but on its frequency spectrum. The device consistsof a hand-held probe containing an acoustic speaker that emits sound burstscomposed of 44 different frequencies, from 1.8 to 4.4 kHz, at 80 dB soundlevel (Figure 3). Using a sensitive microphone and a microprocessor, theEarCheck Pro analyzes the frequency spectra of the reflected sound and presentsthe output as a spectral gradient angle, which corresponds to the probabilityof MEE. The device doesn't require a seal, and measurements can be obtainedin seconds.
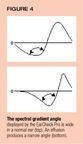
In a normal ear, a significant portion of the sound energy emitted bythe device is transmitted into the middle ear, and little is reflected back,producing a wide spectral gradient angle (Figure 4). In the presence ofan effusion, however, the mobility of the tympanic membrane is restricted,and most of the sound energy is reflected back to the device, producinga narrow spectral gradient angle. Table 3 shows how the spectral gradientangle correlates with five levels of probability of middle ear effusion.The higher the level, the greater the risk of effusion.

Any effusion influences how well a child can hear sounds in the voicefrequency range; consequently, the EarCheck Pro can help identify childrenwith impaired hearing. In 1996, Dr. Combs discovered that of 257 patientshe was monitoring for acute and chronic effusions using a prototype of theEarCheck Pro, 54 failed to pass speech audiometry in one or both ears. Aspectral gradient angle of <95° identified all the children whosehearing threshold was 30 dB or higher. Tympanometry results did not correlatewell with hearing loss, however.11 These findings suggest thata child with a middle ear effusion associated with abnormal spectral gradientangle measurements may have an associated hearing deficit.
In another recent study, SGAR measurements performed on 299 ears provedequivalent to tympanometry for diagnosis of MEE confirmed at the time ofsurgery for insertion of tympanostomy tubes. Among children younger than2 years of age, all those with spectral gradient angles at level 5 (<49°)had MEE at surgery, compared with 15% of those whose spectral gradient angleswere level 1 (>95°).12Another study in 870 children showedthat level 1 spectral gradient angles had a false-negative rate of only3%, while readings at level 5 had a false-positive rate of 8% compared withtympanometry and pneumatic otoscopy. The study also verified that the devicecan obtain readings in ear canals partially occluded by cerumen. It foundthat parents can easily operate the device--a model designed for home useis now available--and that the readings they get correlate well with thosetaken by medical personnel.13
Over the past year, Dr. Schuman has conducted two studies on the professionaland consumer SGAR devices. In one study, 205 children between 6 months and15 years of age undergoing health supervision exams had routine spectralgradient angle measurements with the EarCheck Pro; 26 children (12.7%) hadabnormal SGAR readings. Among children younger than 5 years, 19% had suchreadings. Examination with pneumatic otoscopy revealed that six of the 26childrenwith abnormal readings had AOM and the remaining 20 had OME. None of thesechildren's parents suspected that their child had middle ear disease. Thesefindings suggest that SGAR is useful as a screening tool.
In the second study, parents of children diagnosed with AOM were giventhe consumer device for daily use at home. Children who responded to antibiotictherapy made a rapid transition from their initial SGAR level to a normallevel, generally within a few days of beginning antibiotic treatment. SGARlevels remained unchanged in children with refractory AOM, however. It appearsthat home monitoring of a treated episode of AOM can determine if the infectionis responding to therapy.
The consumer SGAR device can also help parents tell an upper respiratoryinfection from a developing MEE. A child with normal readings is unlikelyto have AOM and can be treated symptomatically, while a child with higher-levelreadings should be evaluated by the physician for OME and AOM.
When the consumer device was released, pediatricians expected a barrageof phone requests for antibiotics, but an informal survey has shown otherwise.Many pediatricians we know who have recommended the consumer device forchildren with a history of recurrent infections report fewer inappropriateoffice visits and fewer phone calls requesting new prescriptions. Parentsseem to like being able to monitor fluid levels during treatment and screenfor fluid when the child has a upper respiratory infection.
The instructions packaged with the device explain that it detects fluidonly and does not show whether the fluid is infected. Still, the deviceis not foolproof. A parent who uses extremely poor technique can obtainfalse negatives, and putting water or ear drops in the canal can producefalse positives.
The EarCheck Pro is powered by two AA batteries and costs about $300.For an additional $300, you can buy an optional base that can be connectedto a printer; this allows you to print out a graphic display of both earmeasurements. The EarCheck Middle Ear Monitor, the consumer version of thedevice, retails in most pharmacies for $100 or less. The consumer instrumenthas a simplified visual display and one-button operation.
Distortion product otoacoustic emissions
When we have a transient ringing in our ears after exposure to a loudnoise, we are experiencing what are known as otoacoustic emissions. Theemissions are the measurable sounds created when cochlea hair cells arestimulated. When sound enters the ear canal, the tympanic membrane vibratesand the middle ear ossicular chain transmits the sound to the cochlea. Thesound waves in the cochlea excite the outer hair cells, which vibrate andconvert the physical energy of the sound wave into electric energy thatgoes to the brain along the auditory nerve. When the sound wave hits theouterhair cells, a backwash of sound energy--otoacoustic emissions--travelsin the reverse direction, from the cochlea and through the middle ear intothe ear canal. In a normal ear, the difference in the intensity of the soundenergy between the stimulating sound and the emissions is about 60 dB, approximatelythe difference between a jackhammer and a whisper. In an abnormal ear, theintensity of the emissions is much weaker than in a normal ear.
Figure 5 depicts a DPOAE device."Distortion product" is theterm used for a special type of otoacoustic emissions that has been shownto be clinically useful because it correlates especially well with puretone audiometry.14 In DPOAE, the sound stimulus is provided bythe simultaneous presentation of two pure tones of equal intensity but differentfrequencies (pitches). A very sensitive microphone is sealed in the probe,which is placed in the ear canal. The device is programmed to listen withina very narrow band at the frequency of the otoacoustic emissions for eachstimulus and to separate the emission from background noise. Responses provideinformation about hearing deficits in these frequency ranges. The instrumentassigns a pass or fail grade for the child's hearing, based on an algorithmstored in memory.

A study of more than 1,000 ears was used to create the algorithm usedby an instrument called AuDx.15 A little larger than a VCR tape,this device is easy to use and tests hearing at 2, 3, 4, and 5 kHz in amatter of minutes. It is programmed to pass or refer at a cut point of 20dB with a sensitivity and specificity of 90% or better. An infant or childwho passes the test is most likely hearing at 20 dB or better, and a childwho fails the test is probably hearing impaired.
Because much of the published research on otoacoustic emissions has beenwith neonates, we tend to think of DPOAE as a screening test only for infantsin the nursery. It can be used with children of any age, however. The technology,which was developed as a test of cochlear function, is sensitive to conductivehearing loss. A large study of pediatric patients has shown that DPOAE canalso provide useful information about middle ear function, since it predictspure tone threshold audiometry results.16Abnormal results donot distinguishbetween a conductive hearing loss and a neurosensory lossor between AOM and OME. A hearing deficit caused by a conductive loss willdisappear along with the middle ear effusion, however. Generally an infantor child who allows tympanometry willallow DPOAE, and the testtakes aboutas long as tympanometry.
DPOAE can be used as a screening test for any age group and during schoolphysicals. It is especially convenient with infants and toddlers, for whomaudiometry is problematic and outside the realm of primary care pediatrics.The technology is most helpful in deciding if surgical management is neededfor bilateral middle ear effusion of more than three months' duration.
The AuDx instrumentcosts about $4,000. It can be purchased with a fastprinter that puts the results on a 2" x 4" self-adhesive label,which can be placed in the patient's chart.
Putting it all together
Pediatricians may want to consider using any or all of these three technologiesas a supplement to pneumatic otoscopy to diagnose and follow the courseof middle ear disease.The algorithm in Figure 6 illustrates how pediatricianscan use office tympanometry, SGAR, and DPOAE audiometry in conjunction withhome SGAR monitoring to manage MEE. We also recommend screening childrenfor occult middle ear diseasewith SGAR (EarCheck Pro) and hearing loss withDPOAE (AuDx) at routine well visits.
Perhaps some futuristic infrared technology will be developed to differentiateAOM from OME. Until then, it is best to think of AOM and OME as a spectrumof disease. Serial determinations with these three technologies can supplythe objective information we need to make the right management decisionfor each patient.

REFERENCES
1. Schappert SM: Office visits for otitis media: United States, 19751990.Advance data from vital and health statistics of the Centers for DiseaseControl/National Center for Health Statistics 1992;214:1
2.Dowell SF, Marcy SM, Phillips WR, et al: Otitis media--principals ofjudicious use of antimicrobial agents. Pediatrics 1998;101(suppl):165
3. Nelson JD: Management of chronic suppurative otitis media: A surveyof practicing pediatricians. Ann Otol Rhinol Laryngol 1988;97(suppl 131):26
4. Klein JO: Protecting the therapeutic advantage of antimicrobial agentsused for otitis media. Pediatr Infect Dis J 1998;17:571
5. McCracken GH: Treatment of acute otitis media in an era of increasingmicrobial resistance. Pediatr Infect Dis J 1998;17:576
6. Rosenfeld RM: What to expect from medical treatment of otitis media.Pediatr Infect Dis J 1995;14:731
7. Stool SE, Berg AO, Carney CT: Otitis media with effusion in youngchildren, in Clinical Practice Guideline Technical Report No. 12, AHCPRPub No 94-0622. Rockville, MD, Agency for Health Care Policy and Research,Public Health Service, US Department of Health and Human Services, July1994
8.Kovunen P, Alho O-P, Ubari M, et al: Minitympanometry in detectingmiddle ear effusion. J Pediatr 1997;131:419
9. Lewis N, Dudgfale A, Canty A,et al: Open-ended tympanometric screening:A new concept. Arch Otolaryngol 1975;101:722
10. Schwartz RH: Diagnostic value of acoustic reflectometry in childrenwith acute otitis media. Pediatr Infect Dis J 1989;8:59
11. Combs JT, Combs MK: Acoustic reflectometry: Spectral analysis andthe conductive hearing loss of otitis media. Pediatr Infect Dis J 1996;15:683
12. Barnett ED, Klein JO, Hawkins KA, et al: Comparison of spectral gradientacoustic reflectometry and other diagnostic techniques for detection ofmiddle ear effusion in children with middle ear disease. Pediatr InfectDis J 1998:17:556
13. Block SL, Mandel E, McLinn S, et al: Spectral gradient acoustic reflectometryfor the detection of middle ear effusion by pediatricians and parents. PediatrInfect Dis J 1998;17:560
14. Spektor Z, Leonard G, Kim DO, et al: Otoacoustic emissions in normaland hearing impaired children and adults. Laryngoscope 1991;101:965
15. Gorga MP, Neely ST, Ohlrich B, et al: From laboratory to clinic:A large-scale study of distortion product otoacoustic emissions in earswith normal hearing and ears with hearing loss. Ear & Hearing 1997;18(6):404
16. Owens JJ, McCoy Mi, Lonsbury-Martin BL,et al: Otoacoustic emissionsin children with normal ears, middle ear dysfunction, and ventilating tubes.Amer Otology 1993;14(1):34
DR. COMBS is in private practice in New Haven, CT. He is also AssistantClinical Professor of Pediatrics at Yale University School of Medicine,New Haven, CT. Dr. Combs participated in the research and development ofthe EarCheck instrument and has received a clinical research grant fromBio-logic Systems Corp. to evaluate the feasibility of using AuDx in privatepractice.
DR. SCHUMAN is Adjunct Professor of Pediatrics at Dartmouth MedicalSchool, Lebanon, NH, and practices pediatrics at Hampshire Pediatrics, Manchester,NH. He is a Contributing Editor for Contemporary Pediatrics. Dr. Schumanreceived a research grant in 1997-1998 from MDI Instruments, Inc. for anoffice study of the EarCheck Pro.