Toddler With Chest Pain, Trouble Breathing, Cough After Heart Surgery
A 3-year-old boy with chest pain and trouble breathing that had developed over the past 24 hours was brought to the emergency department. The parents reported that his most prominent symptom was a cough. The chest pain appeared to worsen with coughing. He had undergone open atrial septal defect repair about 3 weeks before presentation.
HISTORY
A 3-year-old boy with chest pain and trouble breathing that had developed over the past 24 hours was brought to the emergency department. The parents reported that his most prominent symptom was a cough. The chest pain appeared to worsen with coughing. He had undergone open atrial septal defect repair about 3 weeks before presentation. He had been otherwise healthy. Several family members recently had a GI illness. However, the child had no vomiting, diarrhea, or fevers.
PHYSICAL EXAMINATION
Alert, playful, and afebrile child. Heart rate, 103 beats per minute; blood pressure, 88/61 mm Hg; and respiration rate, 28 breaths per minute, with oxygen saturation, 98% on room air. He had notable intercostal retractions and grunting. Cardiovascular examination revealed normal rhythm, with distant heart sounds. Pulses were 2+, capillary refill was less than 2 seconds. He had mild abdominal distention, with a palpable liver edge at 3 to 4 cm. Physical findings otherwise normal.
RADIOGRAPHIC STUDY
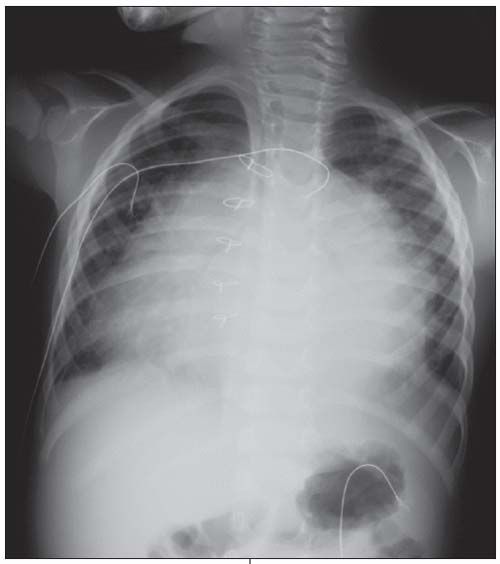
Chest radiograph is shown.
“WHAT'S YOUR DIAGNOSIS?”
Answer on Next Page
ANSWER: POSTPERICARDIOTOMY SYNDROME

Postpericardiotomy syndrome is the development of pericarditis or symptomatic pericardial effusion a few days or weeks after a cardiac operation; atrial septal defect (ASD) repair is a common cause. The chest radiograph shows massive enlargement of the heart in a globular shape, indicative of a large pericardial effusion. In one series of 87 children who underwent surgical closure of an ASD, postpericardiotomy syndrome developed in 6%.1 This syndrome is thought to be an inflammatory or immune-mediated process. Viral infections may serve as a triggering event, although none have been specifically implicated.2
CLINICAL MANIFESTATIONS
Precordial or pleural chest pain is a frequent presenting symptom, although it is not absolute. Young children may initially have anorexia and vomiting, presumably from bowel ischemia.3 Fatigue, sweating, headache, cough, fever, and dyspnea are also common. Physical examination may reveal hypotension and tachycardia. A pericardial friction rub is a classic association; however, this is often absent. The clinician must be alert for signs and symptoms of heart failure in patients who have significant effusion.
Pulsus paradoxus may be present, which should lead to a suspicion of pericardial tamponade. In children without underlying pathology, the volume of the right side of the heart increases with the negative pressure generated during inspiration, and the free wall of the right ventricle stretches to accommodate this volume. In patients with tamponade, the distensibility of the free wall is limited by the fluid in the pericardial sac. The increased volume of the right side of the heart is therefore accommodated by bowing of the interventricular septum leftward. The left ventricle subsequently has decreased volume and is unable to generate normal systolic pressure, leading to decreased cardiac output and a weakened or absent pulse during inspiration.
DIAGNOSTIC STUDIES
A chest radiograph can be suggestive of the diagnosis-as in this patient-especially when the clinical suspicion is high. An ECG can also be helpful. Generalized suppressed voltage is the most common finding. Occasionally, you may also see electrical alternans-beat-to-beat variability in voltage produced by the swinging of the heart in the pericardial fluid.4 An echocardiogram is most specific and can help delineate the degree of effusion. When clinical suspicion is high, diagnostic testing should not delay definitive treatment.
TREATMENT
Treatment involves a 3-pronged approach: intravascular fluid, anti-inflammatory drugs, and drainage. In patients with postpericardiotomy syndrome, accumulation of pericardial fluid leads to reduced diastolic compliance and impaired systemic venous return. In order to overcome this deficit, it is often necessary to give intravascular fluid. Although it seems counterintuitive to give fluid to a patient with respiratory distress from heart failure, the goal is to increase cardiac output.
From a pathophysiological perspective, corticosteroids and NSAIDs can help hasten recovery from postpericardiotomy syndrome.5,6 This is based on the presumptive causative mechanism of an inflammatory or immune-mediated process. In refractory conditions, intravenous immunoglobulin or methotrexate may improve outcomes.7,8
References:
REFERENCES:
1. Jones DA, Radford DJ, Pohlner PG. Outcome following surgical closure of secundum atrial septal defect. J Paediatr Child Health. 2001;37:274-277.
2. Webber SA, Wilson NJ, Junker AK, et al. Postpericardiotomy syndrome: no evidence for a viral etiology. Cardiol Young. 2001;11:67-74.
3. Tanel RE. ECGs in the ED. Pediatr Emerg Care. 2005;21:880-881.
4. Spencker S, Müller D, Mochmann HC. Pericardial effusion and electrical alternans. Resuscitation. 2008;76:163-164.
5. Wilson NJ, Webber SA, Patterson MW, et al. Double-blind placebo-controlled trial of corticosteroids in children with postpericardiotomy syndrome. Pediatr Cardiol. 1994;15:62-65.
6. Horneffer PJ, Miller RH, Pearson TA, et al. The effective treatment of postpericardiotomy syndrome after cardiac operations. A randomized placebo-controlled trial. J Thorac Cardiovasc Surg. 1990;100:292-296.
7. Wendelin G, Fandl A, Beitzke A. High-dose intravenous immunoglobulin in recurrent postpericardiotomy syndrome. Pediatr Cardiol. 2008;29:463-464.
8.Zucker N, Levitas A, Zalzstein E. Methotrexate in recurrent postpericardiotomy syndrome. Cardiol Young. 2003;13:206-208.