Vomiting in infancy: When should you worry?
Emesis in the first year of life, though generally not a cause for concern, can suggest serious disease. Certain signs and symptoms associated with vomiting, particularly the presence of bile, call for imaging studies and other tests.
Vomiting in infancy:
When should you worry?
By Karen F. Murray, MD, and Dennis L. Christie, MD
Emesis in the first year of life, though generally not a cause for concern, can suggest serious disease. Certain signs and symptoms associated with vomiting, particularly the presence of bile, call for imaging studies and other tests.
Vomiting, a common problem in the first year of life, may be innocent and benign, but it also can cause complications or indicate serious underlying disease. To determine what approach to take, pediatricians must distinguish regurgitation from vomiting and be familiar with the differential diagnosis of infant vomiting. You also need to know how to evaluate the baby for the most common causes of vomiting.
Vomiting vs. regurgitation
Vomiting is the forceful expulsion of stomach contents through the mouth. It is mainly a somatic reflex that makes the abdominal and diaphragmatic muscles contract, causing intra-abdominal, and hence intragastric, pressure to increase.1 The older child or adult is considered to have vomited when he or she reports a feeling of nausea, followed by expulsion of stomach contents. With an infant, the nausea is suggested by pallor, sweating, salivation, and tachycardia. True vomiting is preceded by retching, which is caused by the simultaneous inspiratory efforts of the diaphragm and intercostal muscles, closing of the glottis, and contraction of the abdominal musculature. If the retching is prolonged, emesis follows as the distal esophagus relaxes, the cervical esophagus contracts in retrograde fashion, and the mouth opens to discharge gastric contents.2
Regurgitation, in contrast to vomiting, is not associated with nausea or retching. Regurgitation is the passive movement of gastric contents into the esophagus; the contents may then be emitted from the mouth as in vomiting.
Conditions associated with regurgitation
The distinction between true vomiting and regurgitation is particularly relevant in differentiating gastroesophageal reflux (GER) or rumination syndrome from conditions that cause true vomiting.
GER is a very common cause of emission of gastric contents through the mouth in the infant younger than 1 year. The prevalence of GER is difficult to determine because reflux is a normal phenomenon that nearly all infants experience.
Recent studies suggest that activity of the lower esophageal sphincter, a narrow band of tonically contracted smooth muscle at the distal end of the esophagus, may be the primary cause of physiologic GER in children.3,4 Normally this contracted smooth muscle relaxes with the approach of a peristaltic wave in the esophagus, allowing a food bolus to pass into the stomach. In GER, however, the lower esophageal sphincter sometimes relaxes when normal esophageal peristalsis is not occurring, according to the new research. Any condition that interrupts normal gastrointestinal motility may also result in GER.
In the first year of life, regurgitation is the most common symptom of GER. Half of infants from birth to 3 months of age regurgitate at least once a day. This figure increases to about 70% among 4-month-olds, then gradually decreases to 5% among 10- to 12-month-olds.5,6 Many infants experience less reflux when they are in an upright position than when they are lying down. Reflux sometimes is associated with crying or increased activity.
Esophagitis is the most common complication of GER, affecting 5% of infants with GER.5 A baby with esophagitis may be irritable, arch the back during feeds, have behavior problems, avoid food, and grow slowly because of poor caloric intake. Respiratory complications of GER include apnea, pneumonia, cough, and bronchospasm. The mechanism and true association between GER and apnea is not clear, but it is more common in premature than in full-term infants, and bronchospasm or laryngospasm may be a contributory factor. GER and asthma are associated in 40% to 60% of patients.6 In patients with GER and asthma, pulmonary symptoms often improve when GER is treated. Recurrent pneumonia, especially of the right middle lobe, and nocturnal cough are well-known complications of GER, most often caused by aspiration. Aspiration can also result in chronic lung disease. If GER is severe and leads to significant caloric loss because of regurgitation, the infant may gain weight poorly. Food refusal, which is most common when the child has esophagitis, has a similar result.
When GER is uncomplicated by discomfort, feeding impairment, poor weight gain, or respiratory complications, it is usually diagnosed clinically, and further evaluation is unnecessary. The presence of complications or severe symptoms suggests the need for some tests, however. A contrast upper gastrointestinal series must be performed to rule out anatomic causes for recurrent regurgitation or vomiting, such as a large hiatal hernia, esophageal stricture, gastric-outlet obstruction, duodenal web, gastric web, or other anatomic abnormalities. A barium upper GI series is not recommended for evaluating the degree of GER itself. Many infants without symptoms of GER will reflux the barium into the esophagus, and those who do have GER may not have significant reflux during the study itself. Because the barium is inert and does not stimulate motor activity receptors in the duodenum, these studies offer little useful information about the rate of gastric emptying.7
Gastroesophageal scintigraphy has also been used to evaluate GER. A radionuclide such as 99mTc can be added to the infant's diet and the location of the radionuclide monitored with a gamma counter. To obtain reproducible results, this test must be performed by an experienced radiologist. In addition, scintigraphy gives less information than an upper GI series about the degree of GER and is far less sensitive at ruling out anatomic obstruction. Scintigraphy is useful for determining the rate of gastric emptying, which is delayed in about half of children with GER.6 Scintigraphy may document the aspiration of gastric contents into the lungs; the test often produces false negatives, however.
Development of thin flexible pH probes allows prolonged pH monitoring in the esophagus without significantly impairing the child's normal functioning. This monitoring is the best test for quantifying the frequency of GER and rapidity with which the esophagus is able to clear the refluxed acid. To gather the most information from the test, the monitoring is usually conducted over a 24-hour period. These pH probe studies are especially helpful when symptoms suggest GER, but regurgitation is not clinically evident. By recording what the child is doing along with the pH, one can correlate GER episodes with position, eating, or other behaviors. The studies determine how often pH-changing acid reflux occurs, how long the acid remains in the esophagus, and how long it takes the esophagus to clear it; pH probe studies cannot measure the volume of refluxed material or detect reflux episodes that do not change the esophageal pH.
Endoscopy can elucidate an underlying condition affecting the mucosa of the esophagus, stomach, or duodenum that may cause vomiting or increased GER. It generally is reserved for infants suspected of having an underlying inflammatory condition, such as esophagitis, or to define the presence or degree of reflux-induced mucosal injury. Endoscopy does not indicate the degree of GER.
Uncomplicated GER usually resolves by 12 to 18 months of age. Parental reassurance and simple interventions generally are all that are required. The infant should be kept upright after eating, preferably lying flat with the entire body tilted at a 30° angle. Smaller, more-frequent feeds can help some infants, and some benefit from thickened feeds. Breastfed infants may benefit from upright positioning and smaller, more frequent feeds.
Complications of GER warrant medical intervention. Once anatomic causes of recurrent regurgitation have been excluded, acid blockade is generally the first line of therapy. Acid suppression diminishes both gastric fluid volume and acid injury to the esophagus during reflux. It also relieves acid-induced delay in gastric emptying. Table 1 shows the most commonly used antireflux medications, their dosages, and the most common side effects. The newer proton pump inhibitors (lansoprazole and omeprazole) have not yet been approved for use in children. They may prove to be effective and safe medications in infants and children.
TABLE 1
Antireflux medications
The prokinetic agents generally enhance gastric emptying and esophageal peristalsis, and hence clearing, and increase lower esophageal sphincter tone. These drugs generally are reserved for patients who have not responded to standard acid suppressive therapy.8 Cisapride is one prokinetic that has been used to treat GER, but in the wake of reports of heart rhythm abnormalities,8,9 resulting in 80 deaths, the manufacturer of cisapride recently withdrew the agent from the market.
Infant rumination is the repetitive regurgitation of food from the stomach, which the infant then rechews, reswallows, or expels from the mouth.10,11 Rumination has been well described in individuals with bulimia nervosa, the mentally retarded, children, and infants. Infant rumination is a rare but serious condition that may develop when there is no reciprocal interaction between the infant and caregiver. The infant learns to bring up gastric contents into the mouth as a means of self-stimulation and to satisfy needs not being met by the caregiver.12 Onset is typically between 3 and 6 months of age. Much like GER, rumination is effortless and is not accompanied by any apparent distress. In contrast to GER, however, the infant does not ruminate when actively engaged and interested in the immediate surroundings or while sleeping.10,12
Rumination begins with rhythmic contractions of the pharynx, tongue, and abdominal muscles, resulting in regurgitation of gastric contents to or beyond the mouth. The infant mouths, chews, and reswallows whatever remains in the mouth. Accompanying self-stimulatory behaviors may include moving the head, sucking the hand, or making sounds. The physical consequences of rumination can be profound: malnutrition, weight loss, growth failure, dehydration, electrolyte imbalance, and even death.10,12
The diagnosis is based on direct observation of behavior, after excluding physical causes of regurgitation and vomiting. Without rectifying the poor social interactions between the infant and caretaker, failure to thrive will not improve, even with antireflux management, formula changes, or gastrostomy tube feedings. The weight loss and rumination generally respond to appropriate nurturing and sensitive interactions between the caregiver and infant. These are generally best provided by a mother substitute, such as a skilled infant nurse, while the mother is helped to change her own feelings toward herself and her baby and learns how to fulfill her infant's physical and emotional needs.12 This goal must be approached with great care to avoid making the mother feel guilty and angry, further alienating her from the infant.
Conditions that cause vomiting
Vomiting in the first year of life can represent benign self-limited conditions or serious life-threatening diseases. Table 2 lists the most common of both these types of conditions. Noting the presence or absence of bile can help to determine what anatomic abnormality is causing the vomiting and the seriousness of the problem. Any condition that affects the motility of the intestine beyond the ampulla of Vater in the duodenum is likely to result in bilious vomiting, whereas conditions located toward the head of (cephalad to) the duodenal bulb generally cause nonbilious vomiting. Bilious vomiting in an infant should always elicit immediate evaluation since it generally indicates a serious condition that may require rapid surgical intervention. The pediatrician should consider referral to a surgeon or gastroenterologist for any child with bilious vomiting, persistent nonbilious vomiting, or vomiting associated with abdominal pain.
TABLE 2
Conditions that can cause vomiting in infants
Necrotizing enterocolitis (NEC) almost always develops during the neonatal period and is characterized by focal or diffuse ulceration and necrosis of the intestine, most often the distal small bowel and colon. Incidence is one to three cases per 1,000 live births; risk is highest in infants in neonatal intensive care units, 1% to 5% of whom are affected. Although the lower the premature infant's birth weight, the greater the likelihood of NEC, 10% to 35% of affected infants are full term. The cause and exact pathogenesis of NEC are not known, but enteral feeding, intestinal ischemia, and infectious organisms may all play a role.
The classic clinical triad of NEC comprises abdominal distention, bilious vomiting, and blood in the stools. In the full-term infant, the presentation may suggest generalized sepsis, but suspect NEC when GI signs and symptoms predominate. An abdominal roentogram showing pneumatosis intestinalis, air within the wall of the intestine, or air in the hepatic portal system is diagnostic. Air in the peritoneal cavity (pneumoperitoneum) or intra-abdominal fluid is a serious sign calling for immediate surgical intervention.
In most cases of NEC, management is largely supportive, with surgery reserved for infants whose condition becomes worse despite medical management. Stop all oral feedings and have a nasogastric tube placed for suction of gastric contents and intestinal decompression. Obtain cultures of blood, urine, and cerebrospinal fluid and administer broad-spectrum parenteral antibiotics. Obtain abdominal radiographs every six hours to assess disease progression.
Indications for surgery include pneumoperitoneum and cellulitis of the anterior abdominal wall, irreversible metabolic acidosis, or progressive respiratory failure. Resection of the gangrenous bowel is performed with either a primary anastomosis or establishment of a proximal enterostomy.
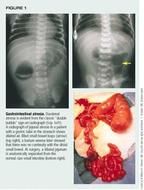
GI atresia/stenosis. Upper GI atresias generally present in the first two days of life. The most common sign is significant vomiting with any feeding. About 85% of duodenal atresias and all complete jejunal atresias cause bilious vomiting. The absence of bilious vomiting does not exclude duodenal atresia in this age group, though, since 15% of duodenal atresias are cephalad to the ampulla of Vater.13 Up to 30% of infants with duodenal atresia have trisomy 21. Excess amniotic fluid may have been a feature of the mother's pregnancy.
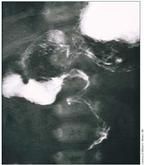
More distal intestinal atresias may become evident a few days later than those in the upper GI tract, signaled by poor feeding, bilious vomiting, and abdominal distention. Meconium usually is not passed until after the first day of life. In all atresias an abdominal roentogram reveals dilated loops of bowel above the obstruction, with little air distally. The classic finding in duodenal atresia is a "double bubble," caused by air dilating the proximal duodenum and the stomach (Figure 1).
Meconium ileus, though not an intestinal atresia, presents in the same way and accounts for 9% to 33% of neonatal small-bowel obstructions.14 About 7% to 25% of infants with cystic fibrosis have this condition, caused by thickened (inspissated) meconium in the distal ileum. The abdominal distention may be evident shortly after birth, and an abdominal roentogram shows dilated distal small bowel filled with material with a speckled or "ground glass" appearance.
Intestinal stenoses sometimes become evident beyond the first few days of life. The vomiting may be more intermittent than in atresias and the distention less severe. Progressively poor feeding and ongoing abdominal distention with bilious vomiting should prompt rapid evaluation with a barium upper GI series with small-bowel follow-through. All atresias and stenoses require surgical intervention.
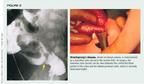
Hirschsprung's disease is marked by the congenital absence of ganglion cells in the submucosal and myenteric plexuses of the distal intestine. The aganglionic segment always is part of the distal rectum and extends proximally varying lengths, most often to the rectosigmoid colon. The area where the aganglionic distal intestine and normally innervated proximal intestine meet is the "transition zone," which can be seen on barium enema as a caliber change between dilated normal bowel and contracted aganglionic bowel.
Within the first weeks of life, infants with Hirschsprung's disease generally develop abdominal distention, constipation, and vomiting, which is usually bilious. A history of delayed passage of meconium is common. Although a barium enema showing a transition zone (Figure 2) is highly suggestive of the diagnosis, a rectal biopsy that reveals a lack of ganglion cells in tissue of the submucosal and myenteric plexuses is required for a definitive diagnosis. Surgical resection of the aganglionic distal segment, generally with later rectal pull-through, is required.
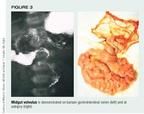
Malrotation and midgut volvulus. During the 10th week of gestation, the intestines return from the umbilical cord to the abdomen, rotating counterclockwise around the axis of the superior mesenteric artery, then becoming fixed to the posterior abdominal wall. The duodenal-jejunal loop rotates behind the artery, and the cecal-colic loop rotates anterior to it, both turning approximately 270° degrees. When this process fails (malrotation), most often the cecum ends up in the right upper quadrant next to the duodenum and the duodenal-jejunal loop is on the right of midline. The resulting mesenteric base is very narrow and centered on the superior mesenteric artery and vein. The poor intestinal fixation and narrow mesenteric base predisposes the intestine to twist about the axis of the superior mesenteric vessels (midgut volvulus), resulting in necrosis of the entire midgut.
Midgut volvulus is most common during early infancy, with 66% of patients presenting the first month and an additional 10% to 20% by 1 year of age.15 The condition can occur at any age, however. The child develops anorexia, abdominal pain, bilious vomiting, and possible fullness in the right upper quadrant. If the midbowel is subject to vascular compromise, the infant may have blood-tinged rectal mucus. A delay in surgical intervention may result in cardiovascular collapse and total midgut necrosis. Malrotation is associated with diaphragmatic hernias, omphaloceles, mesenteric cysts, Hirschsprung's disease, and, rarely, intussusception.14
After the patient is stabilized, the diagnosis is verified by a barium upper GI series showing the abnormal placement of the Treitz's ligament to the left of midline (malrotation) and a corkscrew obstruction (volvulus) in the distal duodenum (Figure 3). A barium enema study may show the cecum in the right upper quadrant, but because the position of the cecum may not be clear or the enema may cause the cecum to move, this diagnostic tool is less sensitive than the upper GI series.
Occasionally, an abdominal ultrasound study that detects an abnormal relationship between the mesenteric artery and vein suggests malrotation. The vein may be to the left of the artery or ventral to it, instead of to its right. This relationship calls for a barium upper GI series to definitively rule out or identify malrotation. Normal positioning of the vessels, on the other hand, is not sensitive enough to exclude malrotation since in 33% of patients with malrotation the superior mesenteric vessels are in a normal position.15
Emergency surgery is required to reduce the volvulus, broaden the base of the mesentery, and fixate the bowel (Ladd's procedure).
Intussusception, the folding of part of the intestine into itself, is the most common abdominal emergency in early childhood and the second most common cause of intestinal obstruction after pyloric stenosis. About 66% of cases are in the first year of life, and the male to female ratio is 3:2. More than 90% of intussusceptions are near the ileocecal valve and most often are ileocolic, though intussusception may occur in other segments of the intestinal tract. The intussusception causes compression of the intramural and mesenteric vasculature, leading to hemorrhage in the bowel wall, ischemia, and ultimately infarction.16 No lead point generally is apparent, though a hypertrophied Peyer's patch (an elevated area of lymphoid tissue on the mucosa of the small intestine), Meckel's diverticulum, polyp, or other lesion may lead the intussusceptum.
In intussusception, the previously well child typically draws up the legs and becomes pale; these are signs of crampy abdominal pain that appear at roughly 20-minute intervals, followed by periods of apathy or lethargy, bilious vomiting, and the passage of bloody "currant-jelly" stools. About 20% of infants have no obvious abdominal pain, and roughly 30% do not pass a "currant-jelly" stool.
Plain roentograms are generally the first diagnostic test in children thought to have intussusception. The X-ray generally reveals a paucity of gas and stool in the colon. In 50% to 60% of children, the test shows a soft tissue mass, the intussusceptum, in the right upper quadrant. In about a quarter of intussusceptions an "adipose rose" is revealed: linear or irregular patches of glowing light representing mesenteric fat within the intussusceptum.15
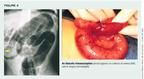
Following the evaluation, a contrast enema is the test of choice since it can be both diagnostic and therapeutic (Figure 4). Contraindications to contrast enema are hypovolemic shock, peritonitis, and intestinal perforation.15 The contrast enema should be performed in the presence of a surgeon and with established intravenous access for fluid resuscitation. Air contrast enema is the preferred treatment for intussusception, which is successfully reduced without surgery in 60% to 80% of patients.15,16 When reduction via enema is not successful, surgery is required.
Infection is one of the most common medical causes of nonbilious vomiting in the first year of life. Acute gastroenteritis is most often the culprit and is usually associated with diarrhea and abdominal pain. In infants, rotavirus is generally the pathogen, but bacterial microorganisms include Salmonella, Shigella, and Campylobacter. Bacterial pathogens, more often than viruses, are associated with bloody diarrhea and high fevers. Giardia lamblia, a protozoan frequently associated with contaminated water and fecal-oral spread in day-care centers, can also cause vomiting and watery diarrhea. Vomiting, in combination with other symptoms, may also be a sign of sepsis, urinary tract infection, or otitis media.
Any bacterial pathogen in the young infant should be treated. Once the child is 3 months old, it generally is not necessary to treat Salmonella unless it is associated with sepsis.
Pyloric stenosis is one of the most common surgical causes of serious nonbilious vomiting in the infant. It results from the profound thickening of the circular muscle of the pylorus of the stomach. Pyloric stenosis occurs in about one in 400 births, with a 4:1 to 5:1 male predominance. Firstborn children seem to be affected more often than other children, and siblings and the offspring of affected individuals are at increased risk of having this condition.13, 7
Infants with pyloric stenosis generally do well until the third to fourth week of life, when they develop progressive nonbilious vomiting and, with time, lose weight. The vomiting becomes projectile, but never bilious. The child is generally very eager to feed, but after eating becomes increasingly fretful and then vomits. As this continues, the infant is apt to develop metabolic hypochloremic alkalosis and may become lethargic. The child who survives without surgery usually recovers spontaneously during the third month of life.
In affected infants, the physician often can palpate an "olive," the swollen pylorus, just to the right of midline of the upper abdomen, if the child's abdominal muscles are relaxed. Ultrasound is the radiographic test of choice for evaluating possible pyloric stenosis. The pyloric muscle is measured directly, making it possible to determine its thickness. Pyloric stenosis is present if the thickness of the muscle equals or exceeds 3.5 mm to 4.0 mm (Figure 5).15 If the presentation is atypical, an upper GI barium radiograph can be beneficial. It generally reveals a filling defect in the distal stomach and duodenal bulb with a string of contrast passing through the narrowed pyloric channel, as long as obstruction of the gastric outlet is not complete.15
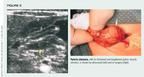
Surgical correction (pyloromyotomy) is the treatment of choice. Metabolic abnormalities must be corrected and the child rehydrated before the curative Fredet-Ramstedt operation is performed.
Cow milk protein allergy, the third most common food allergy after egg and peanut, represents about 8% of all food allergies. In atopic families, incidence may be as high as 23%, but in most countries incidence ranges from 2% to 5%. Incidence is thought to be about 0.5% in breastfed infants.18
Cow milk protein allergy usually becomes apparent in the first year of life, with 30% of patients presenting in the first month and most by the third month. About half of these infants have allergic enterocolitis, manifested by vomiting and diarrhea, frequently with blood and mucus in the stool. Lethargy and dehydration may be part of the picture; these infants do not have elevated specific IgE, and the milk-associated reaction generally resolves by 2 years of age.19 Vomiting can also be associated with cow milk protein allergy that manifests as GER. In fact, it appears that cow milk protein allergy may underlie the GER in about 42% of infants with GER younger than 1 year.18 Eosinophilic gastroenteritis, the eosinophilic infiltration of the mucosa, submucosa, or serosal surface of the intestine, may also present with vomiting (among other symptoms) in the first year of life, but is more often seen in older children. Rarely, cow milk protein allergy appears in an acute form with vomiting, diarrhea, hypovolemic or anaphylactic shock, and, sometimes, angioedema of the lips and laryngeal edema, and acidosis methemoglobinemia.20 A specific IgE serum radioallergosorbent test may be positive. Severe forms of cow milk protein allergy are less readily outgrown than milder conditions.
Cow milk protein allergy is diagnosed by eliminating other conditions in the differential with an upper GI barium series, and, frequently, an overnight pH probe study. Upper endoscopy with biopsies of the mucosa identifies the eosinophilic inflammation associated with cow milk protein allergy, and serum testing for specific IgE or skin testing can be helpful, if positive.
Avoidance of milk and caseinates is the mainstay of therapy. Substituting goat's, ewe's, or mare's milk for cow's milk is not a good idea because cross reactivity to these milks is likely; cross reactivity to soy milk is reported to be as high as 50%. Hypoallergenic milk formulas (such as Good Start) produced by hydrolysis of whey proteins to small peptides are not always therapeutic since the peptides in these formulas retain the reaction-generating epitopes and contain trace amounts of intact b-lactoglobulin. The pre-digested casein hydrolysate formulas (Pregestimil, Nutramigen, and Alimentum), on the other hand, are generally effective substitutes for milk.
Inborn errors of metabolism usually become evident in early infancy. When marked by vomiting, these conditions generally are associated with lethargy, hypotonia or hypertonia, seizures, or coma. Sepsis and gastroenteritis with dehydration can also have this constellation of symptoms, so maintain a high index of suspicion for these conditions in evaluating patients with suspected inborn errors of metabolism. Associated features of metabolic acidosis, hypoglycemia, elevated lactate or pyruvate, hyperammonemia, the presence or absence of ketosis, and a thorough family history, including possible consanguinity, can help elucidate the correct diagnosis.1 Table 3 lists some of the genetic diseases of metabolism that may cause vomiting in the first year of life.
TABLE 3
Genetic metabolic disorders associated with vomiting
Amino/organic acid metabolism
Glutaric acidemia
Isovaleric acidemia
Maple syrup urine disease
Phenylketonuria
Propionic acidemia
Urea cycle defects
Carbohydrate metabolism
Galactosemia
Glycogen storage disease
Hereditary fructose intolerance
Fatty acid oxidation
Medium and long-chain fatty
acyl-CoA dehydrogenase deficiency
Peroxisomal disorders
Adrenal leukodystrophy
Zellweger disease
Putting vomiting in perspective
Vomiting in infancy is common and most often benign. To recognize when it is a symptom of a serious condition, the pediatrician must distinguish between true vomiting and regurgitation, be familiar with the differential diagnosis, and know what tests to perform. The presence of bile in the vomitus indicates that the condition merits an immediate and thorough evaluation.
The authors would like to thank Dr. Robert T. Schaller, Pediatric Surgeon, for supplying the operative photographs and Dr. William D. Winters, Pediatric Radiologist, for providing the radiographs.
REFERENCES
1. Cohen R: Metabolic and infectious disorders associated with emesis in infants. Sem Pediatr Surg 1995; 4:136
2. Miller AD: Central mechanisms of vomiting. Digestive Diseases and Sciences 1999;44:39S
3. Cucchiara S, Bortolotti M, Minella R, et al: Fasting and postprandial mechanisms of gastroesophageal reflux in children with gastroesophageal reflux disease. Dig Dis Sci 1993;38(1):86
4. Mittal RK, Holloway RH, Penagini R, et al: Transient lower esophageal sphincter relaxation. Gastroenterology 1995;109(2): 601
5. Badriul H, Vandenplas Y: Gastro-oesophageal reflux in infancy. J Gastroenterol Hepatol 1999;14:13
6. Faubion WA, Zein NN: Gastroesophageal Reflux in infants and children. Mayo Clin Proc 1998;73:166
7. Hillemeier AC: Gastroesophageal reflux and esophagitis, in Walker WA, Durie PR, Hamilton JR, et al (eds): Pediatric Gastrointestinal Disease, Pathophysiology, Diagnosis, Management, vol 2. St. Louis, MO, Mosby, 1996
8. Vandenplas Y, Belli DC, Benatar A, et al: The role of cisapride in the treatment of pediatric gastroesophageal reflux. J Pediatr Gastroenterol 1999;28:518
9. Hill SL, Pixxi AM, Mobassaleh M, et al: Proarrhythmia associated with cisapride in children. Pediatrics 1998;101:1053
10. Malcolm A, Thumshirn MB, Camilleri M, et al: Rumination syndrome. Mayo Clin Proc 1997;72:646
11. Fabricius ab Aquapendente: Tractatus de Gula, Ventriculo et Intestinis, Padua, Italy, 1618
12. Fleisher DR: Functional vomiting disorders in infancy: Innocent vomiting, nervous vomiting, and infant rumination syndrome. J Pediatr 1994;125:S84
13. Davenport M: ABC of general surgery in children, surgically correctable causes of vomiting in infancy. BMJ 1996;312:236
14. Wesson DE, Haddock G: Congenital anomalies, in Walker WA, Durie PR, Hamilton JR, et al (eds): Pediatric Gastrointestinal Disease, Pathophysiology, Diagnosis, Management, vol 1. St. Louis, MO, Mosby, 1996
15. Weinberger E, Winters W: Abdominal pain and vomiting in infants and children: Imaging evaluation. Compr Ther 1997;23(10):679
16. Wesson DE, Haddock G: Acute intestinal obstruction, in Walker WA, Durie PR, Hamilton JR, et al (eds): Pediatric Gastrointestinal Disease, Pathophysiology, Diagnosis, Management, vol 1. St. Louis, MO, Mosby, 1996
17. Milla PJ: Motor disorders including pyloric stenosis, in Walker WA, Durie PR, Hamilton JR, et al (eds): Pediatric Gastrointestinal Disease, Pathophysiology, Diagnosis, Management, vol 1. St. Louis, MO, Mosby, 1996
18. Moneret-Vautrin DA: Cow's milk allergy. Allergie et Immunologie 1999;31:201
19. Sicherer SH, Eigenmann PA, Sampson HA: Clinical features of food protein-induced enterocolitis syndrome. J Pediatr 1998;133:214
20. Murray KF, Christie DL: Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. J Pediatr 1993;122:90
DR. MURRAY is Director, Hepatobiliary Program, Division of Pediatric Gastroenterology and Nutrition, Children's Hospital and Regional Medical Center, Seattle, WA.
DR. CHRISTIE is Head, Division of Pediatric Gastroenterology and Nutrition, at the same institution.
Karen Murray, Dennis Christie. Vomiting in infancy: When should you worry?. Contemporary Pediatrics 2000;9:81.
Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.
Artificial intelligence improves congenital heart defect detection on prenatal ultrasounds
January 31st 2025AI-assisted software improves clinicians' detection of congenital heart defects in prenatal ultrasounds, enhancing accuracy, confidence, and speed, according to a study presented at SMFM's Annual Pregnancy Meeting.