Ear Anomalies and Neck Sinuses in a Newborn
A newborn male had "cup-shaped ears" and draining neck sinuses that were partially closed by age 3 months. At birth, the child weighed 8 lb 4 oz after a 39-week gestation that was complicated by a single abnormal diabetes screen.
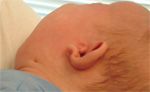
A newborn male had "cup-shaped ears" and draining neck sinuses (Figure 1) that were partially closed by age 3 months.
At birth, the child weighed 8 lb 4 oz after a 39-week gestation that was complicated by a single abnormal diabetes screen. The mother was 35 and the father 31 years at the time of pregnancy. The delivery and nursery stay were uneventful, and the child was breast-fed successfully. The child had mild jaundice and a normal hearing screen.
At age 3 months, the child could fix and follow, lift his head well, roll over from front to back, and respond appropriately to noises. He exhibited excellent growth and was well nourished; his length was in the 50th percentile, weight in the 15th percentile, and head circumference in the 50th percentile. Physical findings were normal except for the bilateral ear anomalies and neck sinuses.
The patient had 2 half-brothers through his mother. One had congenital sensorineural deafness that required the use of hearing aids; the other half-sibling had no hearing defects. A 2-year-old full-brother was healthy except for neonatal hyperbilirubinemia that necessitated phototherapy (bilirubin levels had reached 19 mg/dL). This brother had recently reached a plateau in speech progression; he also had a grade 2 systolic ejection murmur that was investigated by a cardiologist who judged it to be functional.
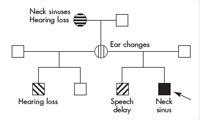
The patient’s mother was healthy. She had a cyst over her left ear that resolved during childhood and mild changes in her external ears. The maternal grandmother also had draining neck sinuses at birth, which necessitated multiple operations because of infections. The grandmother had congenital hearing loss and required hearing aids; she also had congenital right-sided Bell palsy. This grandmother’s 3 sisters and their 2 offspring were healthy, as was the father’s side of the family. Differently patterned shading in the pedigree (Figure 2) reflects the different findings described for the patient and his family members.
What additional studies should be considered For this patient? Also, what disorder(s) should be considered in this patient and his family?
(Answer and discussion begin on the next page.)

ANSWERS:
• Patients with severe or syndromic ear anomalies should undergo audiometry and renal ultrasonography.
• Branchial cysts and/or ear anomalies (including hearing loss) in a patient and his or her relatives suggest a syndrome. The vertical pattern and variable expression in this patient suggest an autosomal dominant disorder, such as branchio-oto-renal syndrome.
Syndromic Ear Anomalies Predict Renal Defects and Hearing Loss
The patient's ear anomalies together with branchial cysts raise the question: what types of ear changes warrant the need for additional studies--including those for kidney defects? Data suggest observation only for those with isolated ear pits or tags and further diagnostic studies for persons with severe or syndromic ear defects (see below). Severe ear anomalies include absent or rudimentary pinnae that may be a sign of anomalies of the ear ossicles or cochleae with consequent hearing loss. Syndromic ear anomalies are those that occur with other branchial arch or pouch derivatives (middle or inner ear, palate, tongue, jaw, neck sinuses, parathyroids, cardiac primordia) or with independent embryonic structures (eyes, urinary tract, limbs, or spine).
Our patient's bilateral cup ears together with branchial sinuses suggested a syndrome and led us to order urinary tract studies. Renal ultrasonography showed a malrotated right kidney and an enlarged left kidney with mild pyelocaliectasis. A voiding cystourethrogram demonstrated vesicoureteral reflux, grade 4 on the left and grade 3 on the right. The patient has had one urinary tract infection with ongoing monitoring and prophylactic therapy. Results of neonatal screening and follow-up hearing testing were normal.
The hereditary implications of branchial anomalies or hearing loss in other relatives focused attention on the patient's full-brother's speech plateau. This brother underwent audiometry, which documented partial sensorineural hearing loss. A renal sonogram showed mild right-sided pelvocaliectasis; the voiding cystourethrogram was normal and urinary cultures were normal.
The mother and the patient's half-brothers will undergo renal ultrasonography.
Why Renal Sonography for an Ear Anomaly?
Such testing is indicated for patients with severe or syndromic defects (including deafness). An association of ear and kidney anomalies is widely assumed but variably supported by epidemiological data. Consensus studies now emphasize that the yield of renal sonography depends on the likelihood that an ear anomaly is associated with other defects including hearing loss (Table).

Examples of conflicting studies include that of Kugelman and associates1 who prospectively studied 108 infants with pre-auricular tags or pits among 17,286 infants (6.2 per 1000 live births). These investigators performed renal sonography on 92 infants and compared them with 95 consecutive healthy infants without anomalies. Abnormal sonographic findings were found in 2 infants with pre-auricular tags (2.2%) and in 3 control infants (3.1%). Mild pyelectasis accounted for the abnormal findings in all but 1 control infant who had a renal calculus. These rates of renal anomalies were similar to those in the general population (all types, 0.2% to 8.1%; significant anomalies, 0.2% to 1.4%) and led to a recommendation against renal ultrasonography.
Kohelet and Arbel2 recommended renal ultrasonography based on their comparison of 70 consecutive infants who had isolated pre-auricular tags with 69 infants who had regurgitation/vomiting or cyanotic spells but no ear anomalies. Urinary tract changes were detected in 6 infants with isolated pre-auricular tags (8.6%) and in none of the control infants; anomalies included hydro- nephrosis in 5 and horseshoe kidney in 1.
Two articles offer resolution of these opposing recommendations.3,4 Both show that additional physical findings and/or a positive family history greatly increase the yield of renal sonography. Among 230 newborns with pre-auricular tags (5.4 per 1000), 10 had other anomalies of the ear and face region and all had hearing impairment (8 conductive, 2 sensorineural or mixed).3 Only 13% of those with isolated ear tags had hearing deficits--all sensorineural and of mild degree. Of the newborns with ear tags and hearing deficits, 78% had a family history of hearing loss. The authors recommend hearing screening in all infants with ear tags; they correlate the presence of additional anomalies, positive family history, and the likelihood of an underlying genetic syndrome.
Wang and associates4 correlate the likelihood of ear anomaly/hearing loss syndrome with that of urinary tract anomalies. They reviewed 42 patients with ear anomalies who underwent renal ultrasonography and found that 12 (29%) also had renal anomalies. Analysis showed that 11 had additional morphological findings that suggested an underlying syndrome, while only 1 had isolated pre- auricular tags. Renal sonograms were recommended only for those with ear anomalies as well as:
•Other malformations.
•A family history of deafness.
•A family history of auricular or renal malformations.
•A maternal history of gestational diabetes.
The data on pre-auricular tags and associated problems are summarized in the Table. Renal ultrasonography in our patient and his relatives would be justified based on the family history of deafness and ear malformations.
Interpreting the Family History
Even in a genetics clinic, the vast majority of patients (about 95%) have an unremarkable family history. General screening questions about the family history are sufficient for primary physicians, followed by documentation of affected relatives. Pedigrees are needed only for those few families with positive responses. Any simple format is adequate; that of males (squares), females (circles), unions (lines), and shaded symbols for symptoms shown in Figure 2is standard. The pedigree in Figure 2 strongly suggests involvement of a single gene to account for 3 affected generations. Multifactorial disorders such as diabetes or schizophrenia have low transmission rates (2% to 3%); those for chromosome disorders are even lower (approximately 1%), barring rare translocations.
Among the single gene or mendelian mechanisms, autosomal or X-linked dominant inheritance is the only one consistent with affliction of both sexes in several generations. Key to this conclusion is the recognition that manifestations may vary among persons with the same mutant gene (variable expressivity). If the ear anomalies, hearing loss, and branchial cysts are recognized as different signs of the same disease, then they present a distinctive vertical pattern. Autosomal recessive inheritance would produce affected siblings with normal parents and previous generations (horizontal pattern of affecteds), while X-linked recessive inheritance would produce affected males related through normal females (oblique pattern of affecteds).
Once the probable mendelian inheritance is recognized, the curious physician can access the Online Mendelian Inheritance in Man database (enter OMIM in browser to find the Web address of http://www. ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM) and then search on disease names or symptoms. A visit to the OMIM database reveals the autosomal dominant branchio-oto-renal (BOR) syndrome as a likely diagnosis in our patient and his family.5
BOR and Other Branchial Syndromes
The presence of neck sinuses, structural defects of the ears (external or internal), and hearing loss should remind the physician that these structures derive from the branchial arches and suggest a possible branchial arch syndrome. Most common is the oculoauriculovertebral spectrum (OAVS)5, which may manifest with unilateral jaw and lower facial atrophy (hemifacial microsomia) to the full Goldenhar syndrome, with accompanying cognate defects plus cardiac, renal, limb, and occasional brain anomalies. Like the overlapping VATER (a combination of vertebral anomalies, anal stenosis, tracheo-esophageal fistula, and radial anomalies) association, OAVS is usually sporadic and frequently associated with maternal diabetes mellitus.
When there is a family history of ear anomalies and hearing loss, other branchial arch syndromes should be considered-including BOR, Townes-Brock, Nager, and Treacher Collins syndromes. It suffices that the pediatrician be aware of correlated branchial anomalies and realize these can be associated with heart, parathyroid, thyroid (which must migrate through branchial structures), skeletal (limbs, spine), and orofacial defects. Evaluation by a geneticist or other specialist is needed.
Although there used to be distinction between branchial and otic defects (BO) versus these plus renal defects (BOR), there is now consensus that these conditions are overlapping manifestations of the same syndrome. This conclusion is strengthened by the fact that many families have had mutations in the EYA1 gene on chromosome 8. EYA1 mutations are detected in 40% of persons with a clinical diagnosis of BOR syndrome, and testing is available in commercial laboratories.
Genetic studies and earlier clinical studies suggest a frequency of BOR syndrome of 1 in 40,000. Those affected have a 6% chance of severe renal anomalies and a 0.5% chance of severe hearing loss.6
The higher rates for renal anomalies or hearing loss summarized in the Tablereflect the fact that at least 20 mendelian and many more chromosomal syndromes include branchial and renal anomalies--some with high frequencies of complications. Among these, the MURCS association of Mullerian, renal, and cervical spine defects has focused attention on the primitive kidney (pronephros) that arises high in the thorax near the branchial apparati.5
Ear Anomalies and Syndromes: Small Contributors to Hearing Loss
Of the 2 to 3 per 1000 children born with hearing loss, 1 per 1000 has profound deafness. Half of all cases have a genetic basis. These figures increase greatly with age. Among those over 80 years, half have hearing loss. This translates into a 10% overall incidence of deafness in the United States. Later-onset hearing loss often reflects environmental factors but can include mendelian diseases, such as hereditary otosclerosis.7
Seventy-five percent of cases of congenital nonsyndromic deafness are autosomal recessive; mutations in the GJB2 gene account for half of these. (This gene encodes a connexin 26 protein crucial for gap junctions between cochlear cells.7) The high frequency of connexin mutations in hearing-impaired infants has prompted consideration of newborn DNA screening. About 20% of cases of nonsyndromic congenital deafness involve autosomal dominant inheritance and only 2% to 3% are X-linked recessive. The diversity of genes in these categories makes DNA testing too insensitive and expensive to be practical at this time.8
Syndromic hearing loss accounts for 25% of congenital deafness and includes a large number of individually rare disorders, such as BOR syndrome. The causative genes are often known, but DNA testing is not routinely available.
Management Summary
Careful physical examination can draw attention to minor anomalies that point to familial and medical risks. Severe ear or other branchial anomalies exemplify this approach by highlighting risks of hearing loss or urinary anomalies and preventing consequences of speech delay or renal failure. Significant genetic influence on deafness and its syndromes draws attention to the family history with potential benefits for affected relatives. DNA testing has high yields in nonsyndromic hearing loss but awaits multigene strategies (eg, comparative genomic hydridization microarray analysis) to become practical for such rare conditions as BOR syndrome.
References:
REFERENCES:
1.
Kugelman A, Tubi A, Bader D, et al. Pre-auricular tags and pits in the newborn: the role of renal ultrasonography.
J Pediatr.
2002;141:388-391.
2.
Kohelet D, Arbel E. A prospective search for urinary tract abnormalities in infants with isolated preauricular tags.
Pediatrics.
2000;105:E61.
3.
Kankkunen A, Thiringer K. Hearing impairment in connection with preauricular tags.
Acta Paediatr Scand.
1987;76:143-146.
4.
Wang RY, Earl DL, Ruder RO, Graham JM Jr. Syndromic ear anomalies and renal ultrasounds.
Pediatrics.
2001;108:E32.
5.
Splinter A, Wilson GN. Oculoauriculovertebral spectrum.
Consultant for Pediatricians.
2007;6:405-407.
6.
Chen A, Francis M, Ni L, et al. Phenotypic manifestations of branchio-oto-renal syndrome.
Am J Med Genet.
1995;58:365-370.
7.
Smith RJ, Robin NH. Genetic testing for deafness--GJB2 and SLC26A4 as causes of deafness.
J Commun Disord.
2002;35:367-377.
8.
Smith RJH. Branchiootorenal syndrome. In:
GeneReviews at Gene Tests: Medical Genetics Information Resources.
Seattle: University of Washington; 1997-2007. Available at: http://www.genetests.org. Accessed August 8, 2007.