A pediatric epidemic: Deformational plagiocephaly/brachycephaly and congenital muscular torticollis
Pediatric healthcare providers are on the front lines to provide early identification and treatment of plagiocephaly/brachycephaly and torticollis for those infants spending more time supine/reclined and less time prone. Here’s why early intervention is so important.
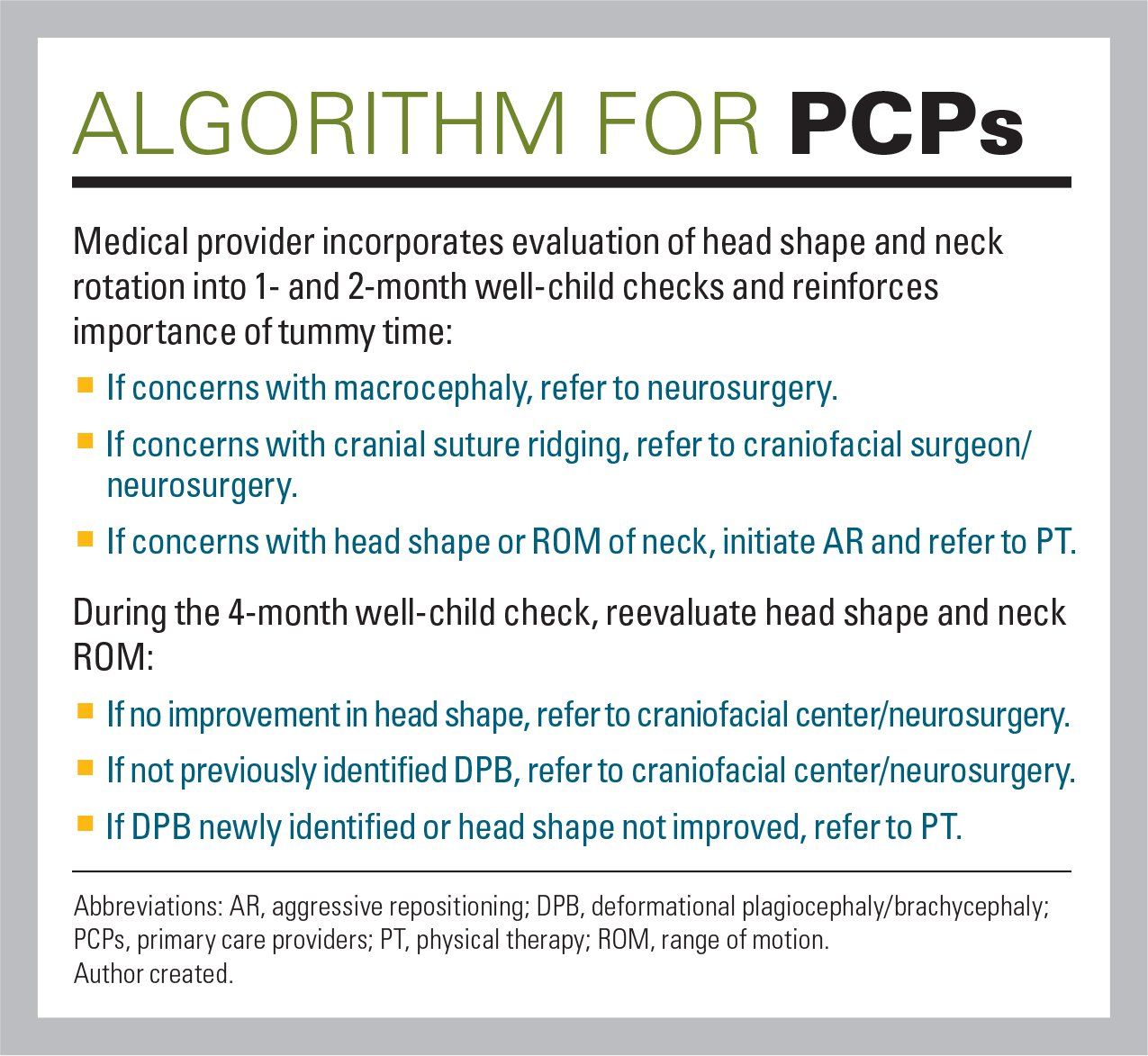
Algorithm for PCPs
Early identification of DPB/CMT

Figures 1-3
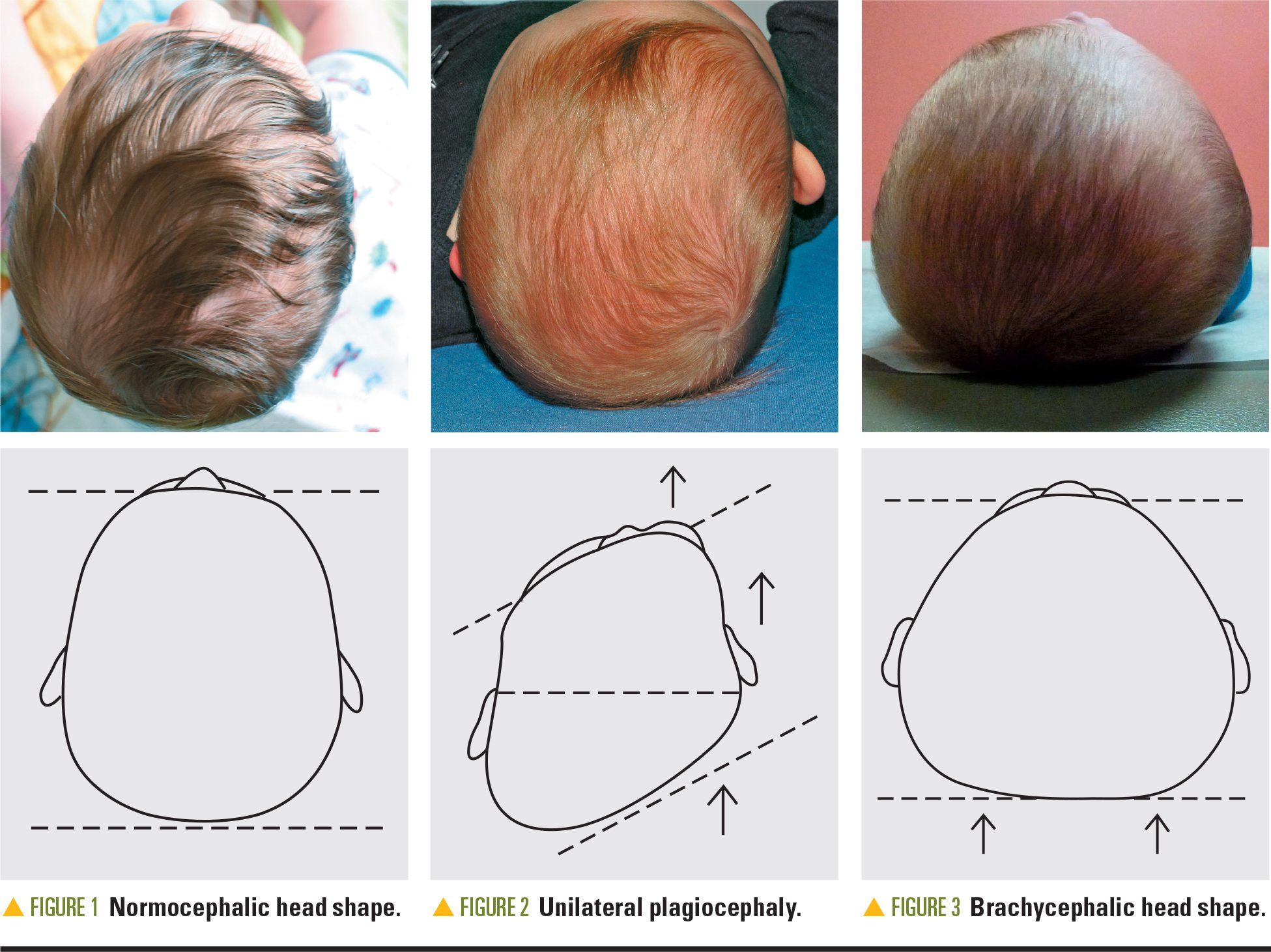
Figure 4A, 4B, 4C
Torticollis: Other associated problems

Figure 5A and 5B
Time Time: Effective Positions

Aggressive repositioning

A pediatric epidemic is sweeping the country. The incidence of infant deformational plagiocephaly and brachycephaly (DPB) and congenital muscular torticollis (CMT) has been on an upward spiral since 1992 when the American Academy of Pediatrics (AAP) instituted the “Back to Sleep” campaign.1 Infants are spending more time supine and in reclined positions day and night and less time prone than in the past.2 We postulate that the widespread increase in DPB and CMT is multifactorial, including frequent use of and/or sleeping in reclined positioners and chairs such as bouncy seats, reclined rockers, swings, and car seats, and dramatically decreased tummy time.
Clinics and pediatrician offices have become inundated with patients exhibiting DPB and CMT, leading to a substantial escalation in costs to the healthcare system. Other repercussions from these diagnoses are an increased need for physical therapy (PT) services and use of helmet therapy (HT), which place additional stress on a family’s time and financial resources. Most community- and government-funded programs (Birth-to-3, early intervention) are strained to accommodate expanding demand for these services.
The aim of this article is to heighten awareness of this epidemic. Pediatric healthcare providers are on the front lines to intervene early in its evolution, allowing them to identify, prevent, and/or treat DPB and CMT with conservative measures. Hopefully, these measures will reverse the process and decrease or eliminate associated exorbitant healthcare costs.
DPB and CMT
Deformational plagiocephaly/brachycephaly occurs from prolonged pressure on the baby’s skull in utero or soon after birth, causing an asymmetric (plagiocephaly) and/or wide (brachycephalic) head shape. The skull is soft and malleable until ossification begins at age 5 to 6 months. When a baby develops a preferred position, the skull will flatten in that area. If the misshapen area is unilateral, the ear, forehead, and cheek will shift anteriorly and impact cosmesis. If the misshapen area is bilateral, the back of the head will widen and may look tall or turricephalic. Incidence of DPB ranges from 18% to 19.7%.3
Congenital muscular torticollis occurs when the sternocleidomastoid (SCM) muscle becomes shortened or restricted unilaterally. The head then turns to the opposite side and/or tilts downward to the same side, resulting in a preferred head position. It becomes difficult for the infant to independently alter head position, and prolonged pressure on the same area occurs when the infant is in a reclined position or sleeping. Also, CMT may develop prenatally due to restricted intrauterine positioning, during delivery, or because of DPB or other external forces. This strains the SCM and surrounding neck musculature causing cervical muscle imbalance and positional preference. The 2 diagnoses usually occur together, creating a synergistic effect.4 Additionally, DPB is strongly associated with CMT-as high as 70% to 95%.3
In the United States, CMT is the third-most common orthopedic diagnosis in infants. Like DPB, its incidence has increased, with a reported range of 0.4% to 1.9% in earlier studies.1,5-9 A rate as high as 16% was reported by 2008.10 Also, CMT has been associated with comorbidities including DPB, facial asymmetries, mandibular asymmetry (MA), developmental hip dysplasia, and gross motor skill asymmetries. Children diagnosed with CMT are treated with skilled PT services to address weakness, range-of-motion limitations, postural deficits, and altered gross motor skill acquisition. A course of PT successfully resolves 90% to 99% of CMT. Surgical intervention (eg, SCM release) is rarely necessary.1,11
Congenital muscular torticollis has an association with MA that can lead to long-term facial asymmetry.1,12 Unilateral ramal height growth restriction, causing jaw asymmetry, results from CMT due to abnormal muscle forces. Mandibular asymmetry can be identified by approximating the mandible to the maxilla. The mandible will cant upward on the side of the head tilt. Physical therapy for the torticollis will address the MA, which is important because MA can affect feeding, especially the ability to achieve latch and adequate suction for breastfeeding.13 Addressing MA early means a greater potential for improvement and resolution. Craniofacial asymmetries, including MA, can become more severe with age when treatment of CMT is delayed or if CMT remains untreated.14
Identifying the problem
Most parents notice the flattening or misshaping of their infant’s head shape between age 1 and 2 months. Parents and primary care physicians (PCPs) do not always recognize CMT because presentation may be subtle. Parents tell us they mention their concern about abnormal head shape and/or positional preference to their PCP but are told it will spontaneously improve once the infant is rolling over and sitting upright. They are discouraged when this does not happen. Although many PCPs believe what they are telling concerned parents about spontaneous improvement, this is typically not the case unless interventions are initiated much earlier in infancy.
In the United States in 2017, 3.8 million babies were born. As noted earlier, incidence of DPB ranges from 18% to 19.7%-about 720,000 infants per year born with DPB.3 About 100 US pediatric plastic surgery/cleft-craniofacial centers each see nearly 100 patients with these diagnoses per month. This does not account for other providers including neurosurgeons or pediatricians. Only about 100,000 of 720,000 infants per year are currently being identified and treated, leaving 86% (620,000) unidentified and untreated. Given long-term, often irreversible, sequelae, this is a serious problem.
Our center’s experience
At our institution, the University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh, Pennsylvania, the cleft-craniofacial center is embedded in the pediatric plastic surgery department. With more than 100 new patients referred to us monthly, most of whom having both DPB and CMT, we developed a multidisciplinary clinic in 2010. Our comprehensive team evaluation and treatment approach includes a nurse practitioner and a physical therapist. With this approach, we are able to institute PT and aggressive repositioning (AR) management at the initial appointment, beginning these conservative measures as soon as possible. Combining these services saves time and money for parents and/or guardians by eliminating the need to schedule a separate PT appointment.
Patients receive a wealth of information during a medical appointment, of which about 80% is not retained once they leave the office.15 To reinforce our recommendations and instruction, we have developed printed patient-education materials to increase understanding and compliance. Handouts include information sheets on DPB and AR techniques as well as brochures about tummy time and torticollis that describe home exercises for CMT.
We also provide community outreach to our regional PCPs and pediatric therapists. Our purpose is to increase awareness of these diagnoses and emphasize the small but critical time frame available to institute conservative measures to treat DPB.
Diagnosis and evaluation
Diagnosis of DPB is determined by physical exam. The cranial exam is performed by having the parent/guardian hold the infant in his/her lap while the nurse practitioner examines the baby from the vertex view (Figure 1). This exam ascertains whether the DPB is unilateral or bilateral. Unilateral DPB most frequently manifests in a parallelogram shape of the head (Figure 2). The flattened side of the head displaces the ear forward anteriorly, causing forehead bossing and fullness of the cheek on the affected side. The orbital opening may be larger on the affected side. Bilateral DPB results in significant brachycephaly (Figure 3). Facial features are not as affected in brachycephalic patients unless both brachycephaly and asymmetry are demonstrated.
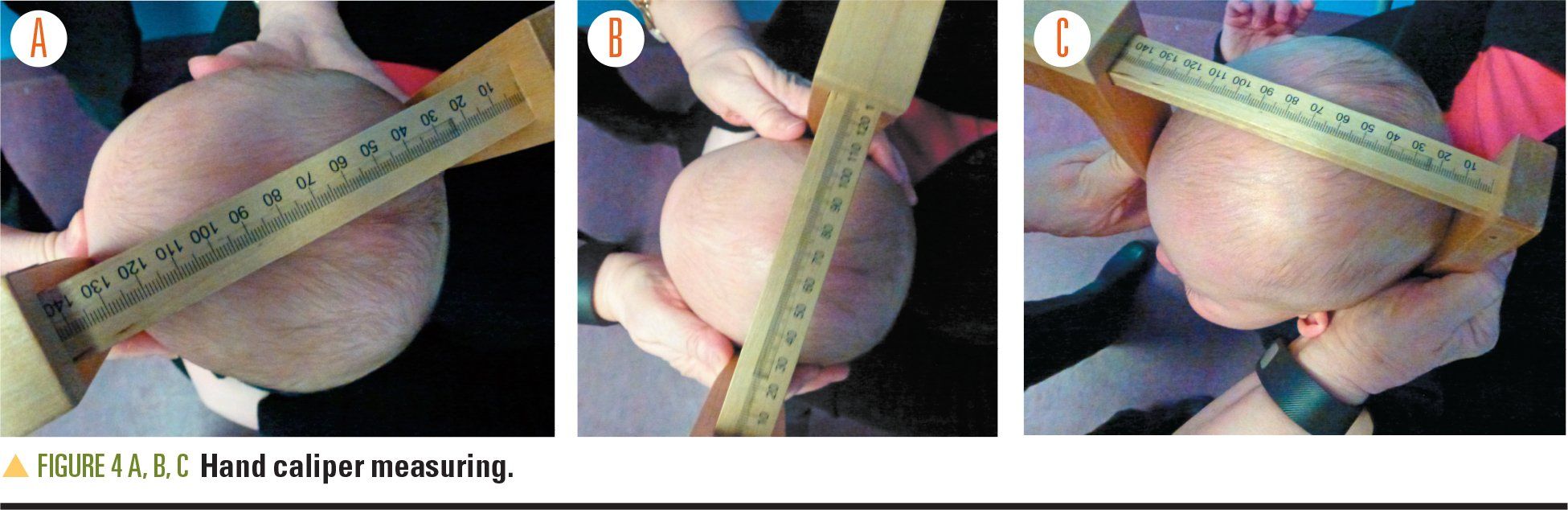
When assessing the cranial vault, a hand caliper is used to measure the cranial index (CI), also referred to as the cephalic ratio, defined as the width divided by the length. The oblique diagonal difference (ODD) is a measurement of the asymmetry of the cranial vault. The CI and ODD provide objective guidelines with which to determine DPB severity (Figure 4). Criteria for cranial vault measurements have not been standardized, but an ODD equal to or greater than 12 mm (and/or confidence index [CI] ≥1.0) has been used to denote DPB as severe. These measurements guide treatment decision-making; eg, mild DPB is treated conservatively with AR and PT.
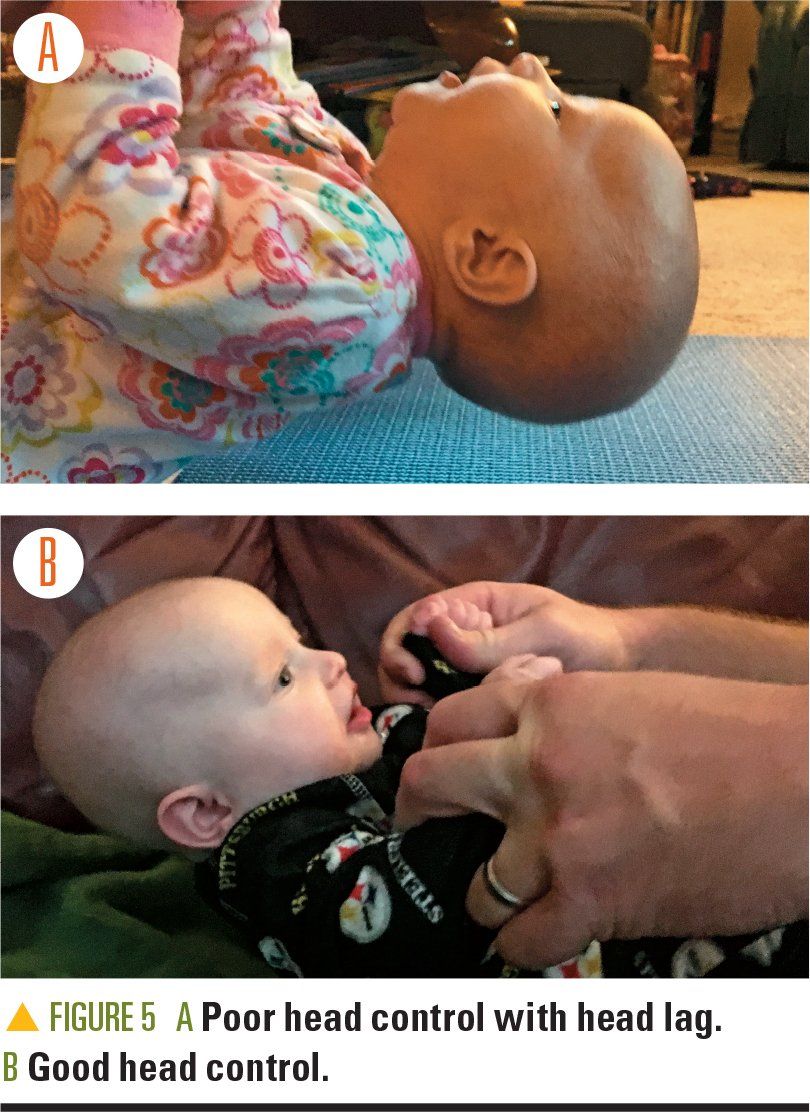
If the baby is aged 4.5 to 5 months or older and has moderate or severe cranial vault measurements, the parent/guardian is offered HT as a choice. The infant must show adequate head and neck control. We verify this developmental achievement by performing a pull-to-sit test to ensure a strong chin tuck is present and there is no head lag (Figure 5). If head lag exists, we recommend increasing tummy time to achieve improved head and neck control and a return visit once adequate head and neck control is achieved. Helmets weigh about 8 ounces, which is a significant weight to place on an infant’s head. Infant safety must be ensured with HT as poor head and neck control combined with the weight of a helmet could potentially compromise respiratory status.
Differential diagnosis
When evaluating these patients, differential diagnoses including craniosynostosis, macrocephaly, hemifacial microsomia, and hydrocephalus must be considered. The occipital frontal circumference is obtained to evaluate for macrocephaly. If there are concerns about head size, we refer to neurosurgery for further evaluation. Cranial sutures are evaluated via palpation for any indication of suture ridging, which can be suggestive of craniosynostosis (fusion or premature closure of skull sutures). If cranial suture ridging is identified and findings are consistent with craniosynostosis, a 3-dimensional computed tomography (3D CT) scan is indicated and HT deferred until it is completed.
Skull x-rays are rarely indicated or helpful. If the 3D CT scan shows craniosynostosis, the patient is referred to a craniofacial surgeon. If the scan does not indicate craniosynostosis, HT can be considered. Some asymmetric facial features observed in children with mild hemifacial microsomia may also be seen in children with DPB and CMT. Children with hemifacial microsomia, however, typically do not present with DPB and/or CMT.
Role of the physical therapist
The physical therapist evaluates the patient’s neck, spine, hips, feet, and provides gross motor skill screening. If the infant has CMT, parents and/or guardians are educated about it, taught exercises to begin immediately, and counseled on initiating PT services via outpatient or early intervention (Birth-to-3). In our state, parents/guardians often opt for early intervention because the state-funded programs do not require insurance and provide the convenience of a physical therapist coming to the home, daycare, or sitter’s home. Such services can take up to a month to initiate, so exercises must start right away. Frequency of PT is weekly or every other week, becoming less frequent as the infant improves. Standard of care for CMT is to continue with PT until the child is walking independently to ensure gross motor milestones are achieved and performed symmetrically.11
Up to 99% of CMT resolves with PT and less than 0% to 1% requires surgical intervention.11 Often CMT manifests with soft tissue restriction of the neck and shoulders, including fibromatosis colli within the SCM in 10% to 50% of cases.1 Families are taught massage for the soft tissue restrictions, which can take months to resolve. Also, a 10% to 14.9% correlation of CMT with developmental hip dysplasia has been documented.1,16,17 The physical therapist undertakes a clinical hip evaluation, and if there are any concerns, the patient is referred to a pediatric healthcare provider or to orthopedics.
Critical importance of tummy time
Initiating awake prone time immediately is crucial in the newborn period. Although parents are well educated about the Back to Sleep campaign to prevent sudden infant death syndrome, they rarely receive sufficient information on the benefits and techniques of tummy time. Our brochure on tummy time reviews techniques for families to use to achieve the goal of 81 minutes by age 4 months.2
Tummy time strengthens the infant’s neck and core and relieves pressure from the head. It is inexpensive, easy to do, and does not require additional products or have associated costs. It would be most beneficial if tummy time were reviewed by the pediatrician or healthcare personnel within the practice during the first newborn appointments. The slogan “Back to sleep, tummy to play” establishes a simple but important message.
Impact of aggressive repositioning
If patients are referred early, between age 0 and 4 months, we initiate the conservative measures of AR and PT. Such techniques are effective then because the skull is soft and malleable until age 5 to 6 months. We strongly support the AAP recommendations of sleeping on a flat, firm surface. Many families have their babies sleeping in reclined chairs, which we believe exacerbates DPB and CMT. In an effort to minimize pressure on the misshaped side of the head, we teach families to use AR. We use a receiving blanket rolled up like a log and tucked behind the affected side of the head, shoulder, waist, and hip when the baby is resting, especially in reclined chairs (eg, bouncy, Fisher Price Rock ‘n Play, or swing chairs). The goal is to minimize reclined positioning and increase upright seating when developmentally appropriate.
Upright chairs for emerging sitters (eg, Bumbo, Fisher Price Sit-Me-Up, Summer Infant, BebePod) are recommended at age 3 to 4 months. These chairs provide necessary back support but allow pressure to be removed from the head. They should be introduced in short intervals, increased as the baby adjusts, and placed on the floor, never on a table or counter due to concern over fall risk. Parents are encouraged to use front carriers in their daily activities to remove pressure from the baby’s head.
Feeding techniques for both bottle-feeding and breastfeeding are provided to support the head and neck, reducing pressure on the affected side of the skull. It is important to reduce laying the baby’s affected side of the head on an arm or items like a pillow. Visual stimulation encourages the baby to look to the opposite side from the DPB.
We use AAP guidelines and state trooper guidelines for car-seat positioning.18 These guidelines require the infant be safely buckled into the car seat and the blanket roll tucked behind the affected side of the head, shoulder, and hip outside of straps and buckles. The family is taught to take the infant out of the reclined car seat upon reaching their destination to prevent further pressure on the affected side of the head. This can be done by holding the baby, using a front carrier, or, when developmentally appropriate, the stroller. We offer a prescription for AR if the baby is enrolled in daycare.
Cranial remolding helmet therapy
Patients return for further evaluation between age 4.5 and 5 months. If conservative techniques have been effective in improving or halting progression of DPB and its severity does not meet criteria for HT, we recommend continuing AR and PT. If between 4.5 and 5 months infants still show significant DPB and meet criteria, we offer HT and many parents agree to it. Again, HT has proved most effective when the skull is still malleable, brain growth is robust, and when initiated prior to the ossification process.19 We inform families that DPB is a functional cosmetic issue because patients need to fit into safety helmets properly when they begin to ride bikes or play helmeted sports. Risk of concussion should not be increased by an ill-fitting helmet due to an abnormal head shape.
Although highly effective, HT can be time consuming and stigmatizing. Often, mothers tell us they feel they have done something to cause this problem. Many cultures are not open to HT. The potential adverse effects of HT include skin issues; ie, rashes, pressure areas, wounds, contact dermatitis, and exacerbation of eczema, seborrhea, or cradle cap. Infants also can become overheated when wearing helmets. Loss of work due to follow-up appointments for adjustments can impact the family. Finally, HT can be very expensive and insurance coverage may be lacking.
Of note, the AAP clinical report from 2011 found no evidence that molding helmets work any better than repositioning for mild or moderate skull deformity.20 Based on studies available then, the best use of helmets for severe deformity is at age 4 to 12 months because of the greater malleability and rapid brain growth. Since 2011, however, newer, larger studies have been completed on the efficacy of HT.
In 2014, a prospective, nonrandomized study recommended treating mild plagiocephaly with repositioning and that HT be the treatment of choice for moderate-to-severe plagiocephaly.21 Another study of 4378 patients found that both conservative treatment and HT were effective.22 Recommendations included repositioning first and HT if repositioning was not effective, or if the baby was older or the condition more severe. A 2015 long-term outcome study comparing those who used helmets versus repositioning found HT provided greater improvement in skull shape than the conservative measure.23 In contrast to other recent findings, a 2016 study found clear improvement in nonsynostotic head deformity treated with a molding helmet and no clear evidence of improvement of absolute measurements in untreated cranial deformity within a 5-year follow-up.24
Although recent review/guidelines from the Congress of Neurological Surgeons (CNS) on HT for patients with positional plagiocephaly indicate AR and PT are important, the report stresses that HT is more effective in reshaping the plagiocephaly.25 It concludes that a body of nonrandomized evidence has shown “more significant and faster improvement of cranial shape in infants with positional plagiocephaly treated with a helmet in comparison with conservative therapy, especially if the deformity is severe, provided that helmet therapy is applied during the appropriate period of infancy.”
We propose, however, that AR and PT initiated early enough have the potential to be as effective as HT in addressing DPB. Patients are rarely referred to us in this early critical timeframe, but when they are, the conservative measures of AR and PT halt or reverse DPB, resulting in substantial improvement and even negating the need for HT. Although it has not been our experience, concerns exist about the overprescribing of HT, but that issue is beyond the scope of this article’s focus.
The epidemic of DPB and CMT has caused a significant financial burden on the healthcare system, especially when HT is used. As the majority of infants are referred too late to institute conservative measures, HT becomes the only option, one that we estimate costs $3.6 million at our center for approximately 900 patients per year.
The United States has over 100 craniofacial centers, and certainly specific costs attributed to this problem vary among them. Nevertheless, referencing our costs for HT as well as plastic surgery consults and PT evaluations and sessions, and multiplying it by 100 centers across the country, the rough gross estimate for costs nationwide quickly reaches more than $1 billion. As well, this estimate does not include costs for patients treated by neurosurgery centers or other providers, missed work, or transportation.
Prevention
Few medical issues occur with this prevalence in otherwise healthy infants, and little attention has been paid to prevention or early treatment in light of the increased numbers of infants with this diagnosis since 1992. Research supporting effective prevention strategies is scant. We recently completed a pilot study approved by the Institutional Review Board that demonstrated support of early referral resulting in less-frequent HT. A Finnish study also has shown that initiating preventive education in the maternity ward from the time infants are born provides significant reduction in the number of infants who develop deformational plagiocephaly or require HT.4
Conclusion
Pediatric healthcare providers are in the best position to identify and manage DPB and CMT. Evaluation of the infant’s head shape and range of motion of the neck should be incorporated into the 1- and 2-month well-child appointments. If any concerns are noted, AR and referral to PT should be initiated immediately.
Lack of intervention or suggesting it will resolve once the baby is rolling and sitting is usually a fallacy. Conservative measures are most effective when the skull is still malleable prior to onset of ossification. If no improvement is observed by the 4-month well-child appointment, referral to a specialist is recommended. Early identification and treatment are critical. They can dramatically improve the patient’s course and provide the momentum to begin to minimize, and hopefully reverse, this epidemic.
References:
1. Karmel-Ross K. Congenital muscular torticollis. In: Campbell SK, Palisano RJ, Orlin MN, eds. Physical Therapy for Children. 4th ed. St. Louis, MO: Elsevier Saunders; 2012:292-312.
2. Dudek-Shriber L, Zelazny S. The effects of prone positioning on the quality and acquisition of developmental milestones in four-month-old infants. Pediatr Phys Ther. 2007;19(1):48-55.
3. Rogers GF. Deformational plagiocephaly, brachycephaly, and scaphocephaly. Part 1: terminology, diagnosis, and etiopathogenesis. J Craniofac Surg. 2011;22(1):9-16.
4. Aarnivala H, Vuollo V, Harila V, Heikkinen T, Pirttiniemi P, Valkama AM. Preventing deformational plagiocephaly through parent guidance: a randomized, controlled trial. Eur J Pediatr. 2015;174(9):1197-1208.
5. Cheng JC, Au AW. Infantile torticollis: a review of 624 cases. J Pediatr Orthop. 1994;14(6):802-808.
6. Cheng JC, Tang SP, Chen TM. Sternocleidomastoid pseudotumor and congenital muscular torticollis in infants: a prospective study of 510 cases. J Pediatr. 1999;134(6):712-716.
7. Cheng JC, Wong MW, Tang SP, Chen TM, Shum SL, Wong EM. Clinical determinants of the outcome of manual stretching in the treatment of congenital muscular torticollis in infants. A prospective study of eight hundred and twenty-one cases. J Bone Joint Surg Am. 2001;83-A(5):679-687.
8. Do TT. Congenital muscular torticollis: current concepts and review of treatment. Curr Opin Pediatr. 2006;18(1):26-29.
9. Tatli B, Aydinli N, Caliskan M, Ozmen M, Bilir F, Acar G. Congenital muscular torticollis: evaluation and classification. Pediatr Neurol. 2006;34(1):41-44.
10. Stellwagen L, Hubbard E, Chambers C, Jones KL. Torticollis, facial asymmetry and plagiocephaly in normal newborns. Arch Dis Child. 2008;93(10):827-831.
11. Kaplan S, Coulter C, Sargent B. Physical therapy management of congenital muscular torticollis: a 2018 evidence-based clinical practice guideline from the APTA Academy of Pediatric Physical Therapy. Pediatr Phys Ther. 2018;30(4):240-290.
12. Kawamoto HK, Kim SS, Jarrahy R, Bradley JP. Differential diagnosis of the idiopathic laterally deviated mandible. Plast Reconstr Surg. 2009;124(5):1599-1609.
13. Wall V, Glass R. Mandibular asymmetry and breastfeeding problems: experience from 11 cases. J Hum Lact. 2006;22(3):328-334.
14. Jeong KY, Min KJ, Woo J, Yim SY. Craniofacial asymmetry in adults with neglected congenital muscular torticollis. Ann Rehabil Med. 2015;39(3):440-450.
15. Bass PF. 3 steps to boost health literacy. Contemp Pediatr. 2018;35(1):13-14.
16. Jackson JC, Runge MM, Nye NS. Common questions about developmental dysplasia of the hip. Am Fam Physician. 2014;90(12):843-850.
17. Kim SN, Shin YB, Kim W, et al. Screening for the coexistence of congenital muscular torticollis and developmental dysplasia of hip. Ann Rehabil Med. 2011;35(4):485-490.
18. American Academy of Pediatrics. Car seats: information for families. HealthyChildren.org website. Available at: https://www.healthychildren.org/English/safety-prevention/on-the-go/Pages/Car-Safety-Seats-Information-for-Families.aspx. Updated August 30, 2018. Accessed January 23, 2019.
19. Kluba S, Kraut W, Reinert S, Krimmel M. What is the optimal time to start helmet therapy in positional plagiocephaly? Plast Reconstr Surg. 2011;128(2):492-498.
20. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128(6):1236-1241. Erratum in: Pediatrics. 2012;129(3):595.
21. Kluba S, Kraut W, Calgeer B, Reinert S, Krimmel M. Treatment of positional plagiocephaly-helmet or no helmet? J Craniomaxillofac Surg. 2014;42(5):683-688.
22. Steinberg JP, Rawlani R, Humphries LS, Rawlani V, Vicari FA. Effectiveness of conservative therapy and helmet therapy for positional cranial deformation. Plast Reconstr Surg. 2015;135(3):833-842.
23. Naidoo SD, Skolnick GB, Patel KB, Woo AS, Cheng AL. Long-term outcomes in treatment of deformational plagiocephaly and brachycephaly using helmet therapy and repositioning: a longitudinal cohort study. Childs Nerv Syst. 2015;31(9):1547-1552.
24. Wilbrand JF, Lautenbacher N, Pons-Kühnermann J, et al. Treated versus untreated positional head deformity. J Craniofac Surg. 2016;27(1):13-18.
25. Flannery AM, Tamber MS, Mazzola C, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines for the management of patients with positional plagiocephaly: executive summary. Neurosurgery. 2016;79(5):623-624.
