Advancements in FDA approvals for pediatric obesity treatment
GLP-1 receptor agonists, used to treat diabetes, are now also being used for obesity in children and adolescents.
Advancements in FDA approvals for pediatric obesity treatment | Image Credit: © monticellllo - © monticellllo - stock.adobe.com.

Pediatric obesity affects more than 14 million children, which is equivalent to 1 in 5 children in the United States. Certain ethnicities have seen a greater rise, with Hispanic ethnicity having the highest prevalence of obesity at 26.2%, followed by non-Hispanic Black ethnicity at 24.8%.1
The American Academy of Pediatrics (AAP) defines obesity as a body mass index (BMI) as greater than or equal to the 95th percentile for age and sex.2 The CDC reported childhood obesity prevalence data from 2017 to 2020 at 12.7% among children aged 2 to 5 years, 20.7% among children aged 6 to 11 years, and 22.2% among children and adolescents aged 12 to 19 years.1
Risk factors for development of childhood obesity include modifiable factors (eg, physical activity levels) and nonmodifiable factors (eg, low socioeconomic status).3 Pediatric obesity has been linked to an increased risk of hypertension, asthma, polycystic ovary syndrome, type 2 diabetes, low self-esteem, depression, and eating disorders.4,5
Caregivers can help prevent childhood obesity by establishing a daily routine, adhering to the American Academy of Sleep Medicine’s age-based sleep recommendations, providing balanced meals, limiting screen time to AAP’s age-based recommendations, and promoting approximately 300 minutes of physical activity weekly.1,6,7 The Kid’s Healthy Eating Plate created by Harvard T.H. Chan School of Public Health can be used as a guide for creating balanced meals.8
Clinicians should query caregivers regarding access to nutritious foods and current support systems in place. Besides sports teams and outdoor activities, indoor physical activity within the home or school or via a community group should be offered to children. Referring social workers for families challenged by living in high-crime areas can increase support, connection, and guidance, as warranted.9
Treatment
Pharmacological treatment may be considered adjunctively when nonpharmacological therapy alone does not achieve the desired results.4 Thus far, the FDA has approved 4 medications for chronic weight management in pediatric populations: orlistat (Xenical),10 phentermine and topiramate extended-release capsules (Qsymia),11 liraglutide (Saxenda),12 and semaglutide (Wegovy).13 This review focuses on the glucagon-like peptide-1 receptor agonists (GLP-1 RAs).
GLP-1 RAs bind to the GLP-1 receptors in the pancreas, brain, and gastrointestinal tract to stimulate insulin release. Additionally, GLP-1 RAs delay gastric emptying, which in turn reduces food intake and promotes a feeling of fullness.14
The FDA granted approval for the first GLP-1 RAs in April 2005 for blood glucose control in adult patients with type 2 diabetes.15 The medication class contains dulaglutide, exenatide, semaglutide, liraglutide, and lixisenatide. Of these, liraglutide and semaglutide are FDA approved for pediatric weight loss.16,17 In December 2020, liraglutide received approval as an adjunctive agent to lifestyle modifications for chronic weight management in pediatric patients 12 years and older with body weight greater than 60 kg and an initial BMI greater than 30 kg/m2 .17 In December 2022, the FDA approved semaglutide as an adjunctive agent to lifestyle modifications for chronic weight management in pediatric patients 12 years and older with an initial BMI equal to or greater than the 95th percentile standardized for age and sex.17 The clinical trial (STEP TEENS; NCT04102189) showed semaglutide was superior to placebo with a change in BMI percentage by week 68 at –16.1% with semaglutide vs +0.6% with placebo. Patients commonly experienced nausea, vomiting, diarrhea, headache, and abdominal pain during the study. Discontinuation of the trial regimen occurred in 5% in semaglutide group and 4% in the placebo group due most commonly to gastrointestinal events.16 Liraglutide (Saxenda) was evaluated in a placebo controlled 56 week long clinical trial with a resulting 2.65% reduction in body weight with liraglutide vs 2.37% increase in body weight with placebo. Gastrointestinal events were common and were the most common reason for regimen discontinuation at 10% in the liraglutide group and 0% in the placebo group.17 Of note, exenatide has been shown to significantly reduce BMI in adolescent patients with severe obesity compared with placebo; however, exenatide is not FDA approved for use in the pediatric population for this indication.18
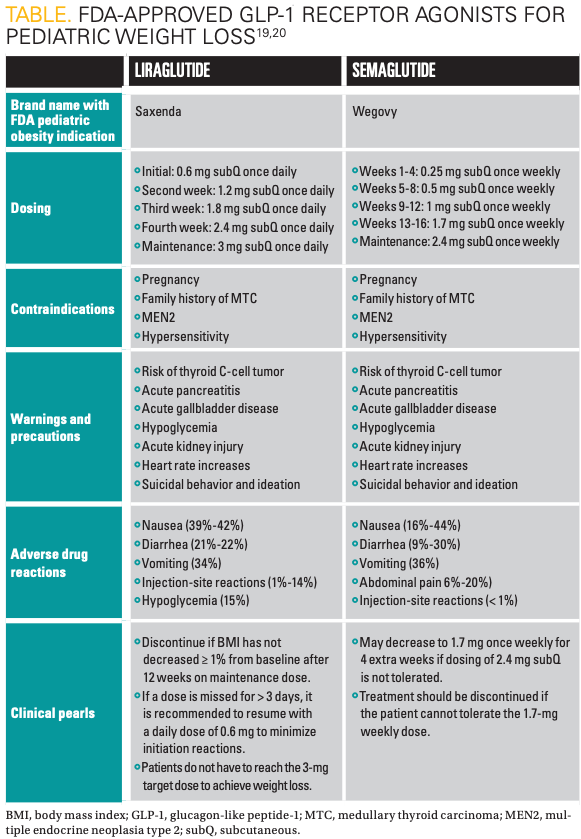
Medication information for liraglutide (Victoza) and semaglutide (Wegovy) can be found in the Table.19,20 GLP-1 RAs are administered via a subcutaneous injection in the abdomen, thigh, or upper arm. Patients and caregivers should be counseled on appropriate medication preparation and injection techniques. Liraglutide and semaglutide products come packaged in ready-to-use pens, whereas exenatide formulations require product manipulation prior to administration, necessitating additional patient education.20,21
No current studies are examining superiority between the GLP-1 RAs in the pediatric population.22,23 More literature is needed to better categorize the expected BMI reduction with each agent. Available studies show up to 76% and 43.3% of adolescent patients reaching 5% or greater BMI reduction with semaglutide and liraglutide, respectively, within the designated study periods.24,25
As mentioned, GLP-1 RAs serve as an adjunctive agent to lifestyle modifications. A meta-analysis conducted by Ryan et al revealed a synergistic effect between the combined use of GLP-1 RAs and lifestyle interventions, which resulted in greater reductions in blood pressure, hemoglobin A1c levels, and weight compared with using GLP-1 RAs alone.25 Other considerations when choosing between products may include cost and insurance formulary.
Conclusion
Pharmacological treatment can be offered adjunctively when desired results are not achieved through nonpharmacological measures alone. Semaglutide and liraglutide are 2 FDA-approved medications for this indication that can be considered for treatment.
For more from the July, 2023 issue of Contemporary Pediatrics®, click here.
References:
- Stierman B, Afful J, Carroll MD, et al. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files—development of files and prevalence estimates for selected health outcomes. CDC. June 14, 2021. Accessed May 18, 2023. https://stacks.cdc.gov/view/cdc/106273
- Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640. doi:10.1542/peds.2022-060640
- Chi DL, Luu M, Chu F. A scoping review of epidemiologic risk factors for pediatric obesity: implications for future childhood obesity and dental caries prevention research. J Public Health Dent. 2017;77(suppl 1):S8-S31. doi:10.1111/jphd.12221
- Smith JD, Fu E, Kobayashi MA. Prevention and management of childhood obesity and its psychological and health comorbidities. Annu Rev Clin Psychol. 2020;16:351-378.doi:10.1146/annurev-clinpsy-100219-060201
- Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251-265. doi:10.1016/j.mayocp.2016.09.017
- Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020-2028. doi:10.1001/jama.2018.14854
- Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12(6):785-786. doi:10.5664/jcsm.5866
- Kid’s Healthy Eating Plate. Harvard T.H. Chan School of Public Health. 2015. Accessed May 18, 2023. https://www.hsph.harvard.edu/nutritionsource/kids-healthy-eating-plate/
- Ayala-Marín AM, Iguacel I, Miguel-Etayo P, Moreno LA. Consideration of social disadvantages for understanding and preventing obesity in children. Front Public Health. 2020;8:423. doi:10.3389/fpubh.2020.00423
- Xenical. Prescribing information. H2-Pharma LLC; 2022. Accessed June 7, 2023. https://xenical.com/pdf/PI_Xenical-brand_FINAL.PDF
- Qsymia. Prescribing information. Vivus LLC; 2022. Accessed May 18, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022580s021lbl.pdf
- Saxenda. Prescribing information. Novo Nordisk Inc; 2022. Accessed June 7, 2023. https://www.novo-pi.com/saxenda.pdf
- Wegovy. Prescribing information. Novo Nordisk Inc; 2023. Accessed June 7, 2023. https://www.novo-pi.com/wegovy.pdf
- Zhao X, Wang M, Wen Z, et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne). 2021;12:721135. doi:10.3389/fendo.2021.721135
- Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96(1133):156-161. doi:10.1136/postgradmedj-2019-137186
- FDA approves weight management drug for patients aged 12 and older. News release. FDA. December 4, 2020. Accessed May 18, 2023. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older
- FDA approves once-weekly Wegovy injection for the treatment of obesity in teens aged 12 years and older. News release. Novo Nordisk. December 23, 2022. Accessed May 18, 2023. https://www.novonordisk-us.com/media/news-archive/news-details.html?id=151389
- Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167(4):355-360. doi:10.1001/jamapediatrics.2013.1045
- Saxenda. Prescribing information. Novo Nordisk Inc; 2022. Accessed June 7, 2023. https://www.novo-pi.com/saxenda.pdf
- Wegovy. Prescribing information. Novo Nordisk Inc; 2022. Accessed June 7, 2023. https://www.novo-pi.com/wegovy.pdf
- Murvelashvili N, Xie L, Schellinger JN, et al. Effectiveness of semaglutide versus liraglutide for treating post-metabolic and bariatric surgery weight recurrence. Obesity (Silver Spring). 2023;31(5):1280-1289. doi:10.1002/oby.23736
- Rubino DM, Greenway FL, Khalid U, et al; STEP 8 Investigators. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138-150. doi:10.1001/jama.2021.23619
- Weghuber D, Barrett T, Barrientos-Peréz M, et al; STEP TEENS Investigators. Once weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245-2257. doi:10.1056/NEJMoa2208601
- Kelly AS, Auerbach P, Barrientos-Perez M, et al; NN8022-4180 Trial Investigators. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117-2128. doi:10.1056/NEJMoa1916038
- Ryan PM, Seltzer S, Hayward NE, Rodriguez DA, Sless RT, Hawkes CP. Safety and efficacy of glucagon-like peptide-1 receptor agonists in children and adolescents with obesity: a meta-analysis. J Pediatr. 2021;236:137-147.e13. doi:10.1016/j.jpeds.2021.05.009
