Anaphylaxis essentials for infants
The American Academy of Pediatrics’ (AAP) has updated its Allergy and Anaphylaxis Emergency Action Plan for the treatment of infants at risk for an allergic emergency.
Table 1
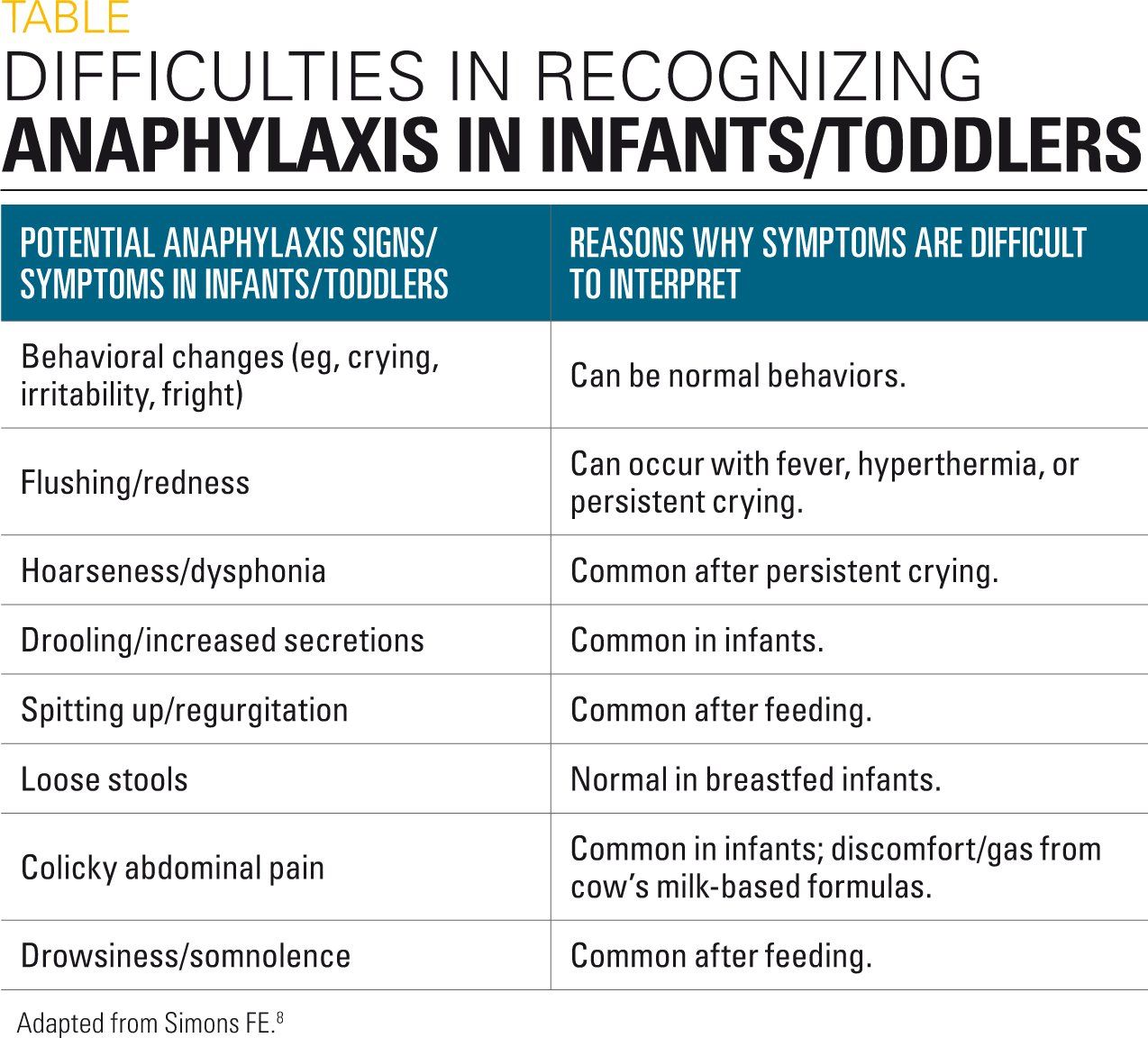
Figure 1
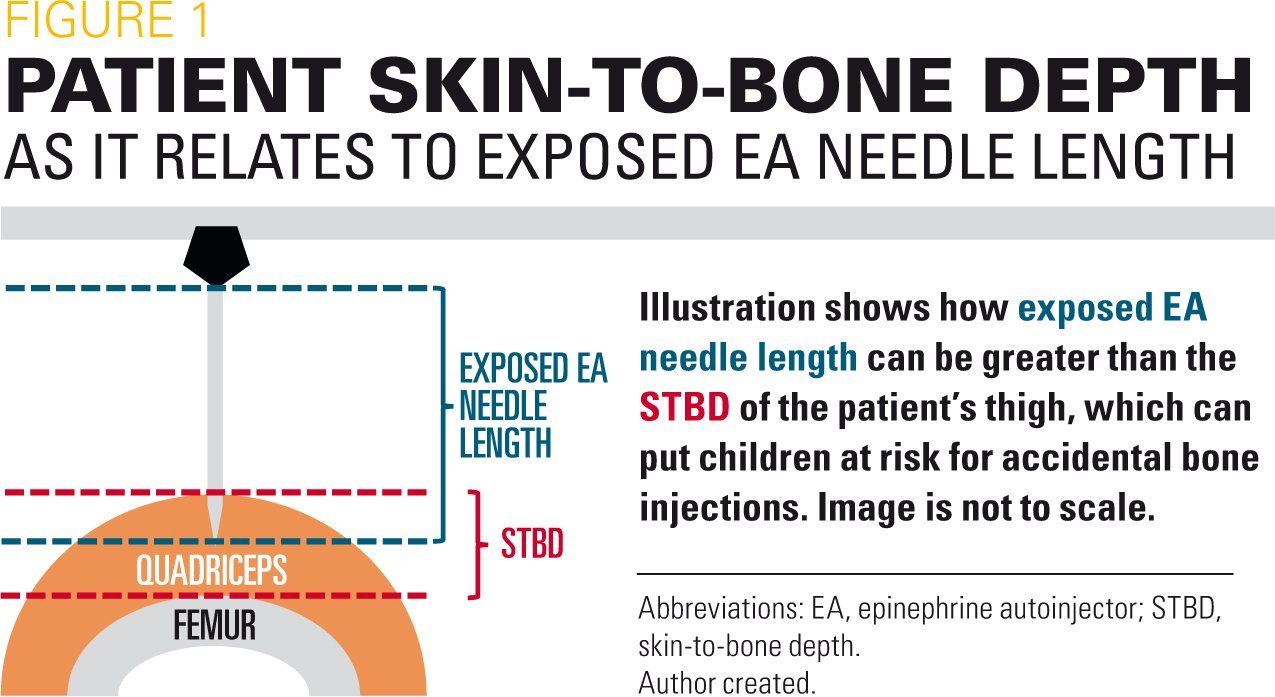
Figure 2
Food allergy and anaphylaxis in infant/toddler-aged children are growing trends. Food allergy is the leading cause of anaphylaxis in infants and toddlers,1 and the most common food triggers causing anaphylaxis in infants are cow’s milk, egg, and peanut.2,3 Although far less is known about the prevalence and nature of anaphylaxis in the infant/toddler population compared with older children and adults, over the last several years food allergy and food-related anaphylaxis has continued to grow among the infant/toddler age group.2,4
For instance, between 1997 and 2007, food allergy in children increased by 18% in the United States.4 From 2007 to 2012, another study found a 50% increase in episodes of food-induced anaphylaxis presenting to the emergency department (ED).5 A more recent study showed that the increase in ED visits for anaphylaxis in children aged 0 to 5 years between 2005 to 2014 was 129%.6 It is likely that a child’s first reaction will occur when aged younger than 1 year. Therefore, these children are more likely to be brought to the ED and hospitalized.5 This becomes an opportunity to recommend referral to allergy specialty providers and deliver education on anaphylaxis.
There appears to be an upward trend in food-induced anaphylaxis in this population, which continues to be a problem in the United States. Obstacles to rapid treatment in this population have been identified as a lack of recognition of reaction severity, not having epinephrine available, and parent/caregiver fear of giving epinephrine.7 Progress toward identification, accurate diagnosis, and management of anaphylaxis in infants and toddlers will require that pediatric healthcare providers recognize important barriers to appropriate management.
Is it anaphylaxis?
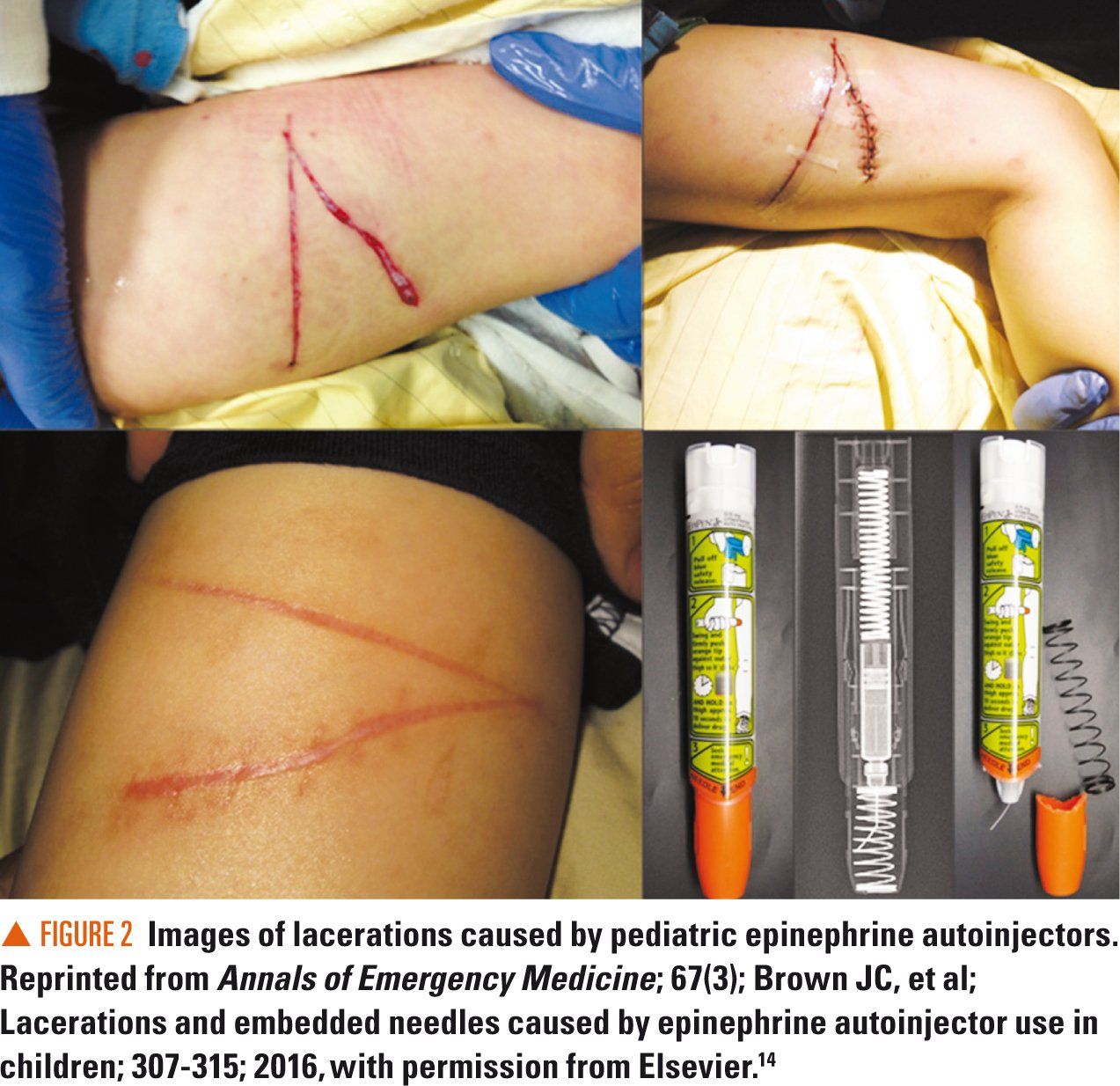
Accurate diagnosis of anaphylaxis requires that healthcare providers understand the possible presentations of anaphylaxis in infants and toddlers. A unique aspect of anaphylaxis in this age group is that a reaction itself may be difficult to recognize (Table), and therefore diagnosis can be challenging. This is because some of the symptoms of anaphylaxis (eg. vomiting, throat itching/tightness, gastrointestinal changes, hives, or hypotension) can manifest in an infant/toddler as regurgitation, irritability and fussiness, drooling, inconsolable crying, apparent contact rash from food, and lethargy/sleepiness.
Unfortunately, the latter behaviors can be perceived as normal in healthy children even during an allergic reaction.3 Another unique aspect of anaphylaxis in infants and toddlers that influences the ability to recognize a reaction is that they are not able verbalize their symptoms;8 in particular, symptoms such as feeling faint, throat tightness, or the feeling of impending doom.3 These symptoms, which can be described by older children and adults, are sometimes critical to understanding the severity of a life-threatening reaction and the need to act.
Although hives and vomiting are more commonly described during anaphylaxis in infants and toddlers,9 the precise symptomatology, or criteria for diagnosis for that matter, of anaphylaxis for these children is not well defined.3 Anaphylaxis may also be characterized by cough, wheeze, stridor, lethargy/drowsiness, and/or persistent gastrointestinal symptoms. In some cases, persistent vomiting may be the only sign.
A 2011 study reported the symptoms that infants presented with in the ED.9 The initial presentation of anaphylaxis in infants is cutaneous in 98% (95% confidence interval [CI], 94% to 100%), involves the respiratory system in 59% (95% CI, 47% to 71%), and involves the gastrointestinal system in 56% (95% CI, 44% to 67%) of cases. In some cases, changes in skin color and subtle behaviors such as drooling and scratching can be signs of an emerging, serious allergic reaction.9 Cardiovascular symptoms were reported as infrequent.9
In real-world settings, the ability to distinguish normal infant/toddler behaviors, or contextual incidents (eg, contact rash on the face from food or vomit) from the manifestations of anaphylaxis are important for accurate diagnosis and treatment. For example, a contact rash on a child’s face after vomiting in the crib is different from hives that appear on the face after ingesting a possible allergen. However, some parents may suspect an allergic reaction. If hives appear on a child’s back, arms, or legs following ingestion of a potential allergen, then an allergic reaction should be suspected (ie, contextual and temporal indicators).
The addition of wheezing, trouble breathing, retching/repeat vomiting, or cyanosis to cutaneous symptoms are clear indicators that an anaphylactic reaction is happening. All patients are different, so considerations of core symptoms in any order, their severity, and the level of concern demonstrated by the parent or caregiver at the time of the reaction can help inform anaphylaxis treatment decision making.
Treatment of anaphylaxis in infants/toddlers is different
Epinephrine is the mainstay of treatment for anaphylaxis regardless of age10,11If anaphylaxis is certain, epinephrine given by intramuscular injection to the mid-outer thigh at a dose of 0.01 mg/kg is currently recommended.3,10 In the absence of a more ideal therapeutic option, pediatric epinephrine autoinjectors (EAs) containing a 0.15 mg dose of epinephrine, and indicated for children weighing 33 lb to 66 lb, have been the only option for infants and toddlers in community settings. However, there is some evidence that suggests these EAs may not be ideal for these young children.
It has been demonstrated that the exposed needle length (0.5 inch) in available 0.15 mg pediatric EAs could unintentionally strike the bone in some children weighing less than 33 lb (Figure 1).12,13 In one study, 29% of children weighing less than 33 lb (n=100) had a skin-to-bone distance (STBD) by ultrasound that was less than 0.5 inch, which would put these children at risk for an unintentional bone strike.12 In a subgroup of children weighing less than 22 lb in this study, 60% (n=25) were at risk for this type of injury.
In a more recent study, 43.1% of patients (16.5 lb to 33 lb; n=51) would be at risk of an accidental bone injection if using one of the available 0.15-mg pediatric EAs.13 This study determined that the appropriate needle length for children weighing 16.5 lb to 33 lb would be 0.28 inch to 0.31 inch. Furthermore, injuries (lacerations and embedded needles) have been reported in infant/toddler-aged children using pediatric 0.15-mg EAs (Figure 2).14 As a result, the prescribing information of pen-style EAs was updated in 2016 to include language on ensuring that a child’s leg is held firmly during an injection.
To address these unmet needs (ie, lower dose and shorter needle length), a new EA containing an epinephrine dose of 0.1 mg and with a 0.29-inch needle was recently approved by the US Food and Drug Administration (FDA) for children weighing 16.5 lb to 33 lb This new EA (Auvi-Q; kaléo Incorporated; Richmond, Virginia) is the only FDA-approved device engineered for infants and toddlers weighing 16.5 lb to 33 lb.
Summary
Available therapeutic options used to treat infants with anaphylaxis may not be ideal, but have been prescribed because of the lack of a more appropriate, FDA-approved option.
In August 2017, Contemporary Pediatrics highlighted the release of the first-ever written Allergy and Anaphylaxis Emergency Plan published by the AAP.15,16 In response to the need for individualized treatment of infants, the AAP has updated this plan to include the 0.1-mg dose EA for children weighing 16.5 lb to 33 lb. The updated plan can be downloaded at: https://www.aap.org/en-us/Documents/AAP_Allergy_and_Anaphylaxis_Emergency_Plan.pdf.
MEDICAL WRITING AND EDITORIAL ASSISTANCE WAS PROVIDED BY SEAN M. GREGORY, PHD, HYBRID HEALTHCARE COMMUNICATIONS LLC, AND FUNDED BY KALÃO, WHICH HAD NO ROLE IN THE DEVELOPMENT, REVIEW, OR FINAL APPROVAL OF THE MANUSCRIPT.

Omalizumab outperforms oral immunotherapy in treating multi-food allergy
March 27th 2025A new clinical trial has found that omalizumab (Xolair; Genetech, Novartis) is more effective than oral immunotherapy (OIT) in treating multi-food allergy in individuals with severe allergic reactions to small amounts of common food allergens.