HCPs can play an important role in addressing the US infant formula shortage
When breastfeeding is not an option, caregivers will turn to infant formulas to meet their child’s nutritional needs.
HCPs can play a role in addressing the US infant formula shortage | Image Credit: © 279photo - © 279photo - stock.adobe.com.

In results from a 2020 survey conducted by the CDC, 45.3% of infants in the United States were exclusively breastfed at the age of 3 months, with this percentage dropping to 25.4% by the age of 6 months.1 The World Health Organization and the US Dietary Guidelines for Americans recommend that all infants should exclusively receive breast milk until the age of at least 6 months.2,3 Caregivers can begin incorporating other solid and liquid foods at this 6-month mark; however, children should continue breastfeeding until the age of 2 years. The CDC reports that 20.8% of breastfed infants receive additional formula supplementation within the first 2 days of life, which can be attributed to a multitude of factors, including lactation issues, scheduling conflicts, and stigma.1 When breastfeeding is not an option, caregivers will turn to infant formulas to meet their child’s nutritional needs.
Infant formulas contain important macronutrients such as carbohydrates and proteins, which are crucial to growth and development.4 In addition to the macronutrients, the infant formula contains micronutrients such as vitamin D, iron, and zinc. Vitamin D is a fat-soluble vitamin essential for the development of bones and the prevention of conditions such as rickets by regulating calcium and phosphorus levels in the body.4-6 Iron is required for hemoglobin production and tissue oxygenation, with low levels of iron causing anemia and failure to thrive.4,7 Zinc is a mineral that contributes to growth and immune function, with deficiencies leading to growth failure and skin rashes.4,6 Due to potential complications, it is important for all pediatric patients to meet their nutritional requirements. Unfortunately, when infant formula shortages occur, access is limited, leading to varying conservation efforts or work-arounds that put the infant at risk for the deficiencies and complications noted previously.
Infant formula shortages can be a direct result of supply chain issues, natural disasters, and/or recalls.8,9 One such example is the infant formula shortage in the United States in April 2022. Several brands of powdered infant formulas were found to be contaminated with Cronobacter sakazakii, prompting a nationwide recall due to the risk of sepsis and meningitis after ingestion.10 Another more recent example is the infant formula recall in the United States in December 2023, also due to Cronobacter sakazakii contamination.11 Health care providers (HCPs) play an important role in shortages, as they can provide education and resources to caregivers in need. The resources that HCPs can provide include but are not limited to comparative formulations, imports, proper feeding practices, and milk banks.
The North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) has infant formula comparison guides that clinicians can use to make safe interchange recommendations.12,13 For example, NASPGHAN states that Similac Alimentum powder (Abbott) is interchangeable with Extensive HA (Gerber) for infants who require extensively hydrolyzed or hypoallergenic formula.13 HCPs can also recommend alternative formulations such as liquid concentrate or ready-to-feed formulations and provide appropriate mixing instructions, as this varies based on the product formulation.14 For example, powder and liquid concentrate formulations require mixing with water in varied ratios whereas ready-to-feed formulations require no mixing. HCPs should also counsel the family on a use-by date after mixing or opening the formulation, as these vary between the different formulations.15 However, it is imperative to note that cost fluctuates between the different formulations, and financial barriers should be considered.
Furthermore, families can seek imported formulas that are authorized by the FDA and available in US stores as an alternative.12 Initially, the FDA temporarily approved imported formulas that met the nutritional requirements but may not have met the labeling requirements of products in the United States.16 Manufacturers who received temporary approval during the initial shortage have since been provided guidance on labeling requirements in order to continue to market their product in the United States. Imported infant formulas are approved by the FDA with a close examination of the nutrients provided by the individual formula and compared with those required by US standards. Clinicians are encouraged to access the FDA’s website, which provides recommendations for appropriate substitutions when switching to an imported infant formula.13,17 Third-party imported formula websites may sell products that are not FDA authorized and do not undergo the scrutiny necessary to mirror the nutritional values of US standards. Accessing and/or ordering from these websites should be avoided.4,18 The FDA provides advice to avoid counterfeit infant formulas by confirming the lot number and use-by dates on the package, checking for damage or label tampering, or calling the manufacturer’s toll-free line.19 If caregivers have used a specific product in the past, they should look out for discoloration and changes in smell or taste.
If an FDA-authorized imported infant formula is chosen, there are unique considerations. There may be unfamiliar language in the patient-facing directions, such as using the word “teats” for the nipple of the baby bottle.15 Additionally, the definition of a special infant formulation may vary based on the country of origin when compared with that of the United States. Furthermore, labeling may contain different languages that may not be readily translated. Imported products may use the metric system, requiring unit conversions and subsequent relay of this information to the caregiver. This is a key counseling point, as mixing the formula incorrectly may lead to electrolyte imbalance, seizures, and poor weight gain.15,16
Human milk banks are an option for caregivers if alternative formulas cannot be obtained. HCPs can provide caregivers a contact number for a local certified human breast milk donation center through the Human Milk Banking Association of North America.18 Human milk donors are thoroughly screened prior to donation.Purchasing human milk from the internet or social media sites should be avoided, as the milk is not adequately screened or regulated and could unintentionally expose the infant to infectious diseases, illicit drugs, and chemical contaminants.12
Cow’s milk is normally not recommended for children until they are 12 months or older due to nutritional differences, such as low levels of bioavailable iron and higher amounts of protein.20 If both human milk and infant formulas are unavailable, the American Academy of Pediatrics (AAP) recommends that infants older than 6 months consume cow’s milk for no more than 1 week.21 Iron supplementation can be given to infants under the supervision of a physician in the form of pediatric drops if they are younger than 6 months.8 Infants can be introduced to solid food at approximately 6 months of age, so it is important to introduce iron-rich foods or cereals to avoid iron deficiency.7,20 There are 2 sources of iron: heme and nonheme iron.7 Heme iron is available in red meat, seafood, and poultry. It is more easily absorbed by the body than nonheme iron. Nonheme iron is available in iron-fortified infant cereals, tofu, and beans. Moreover, goat’s milk is not approved for infants in the United States and plant-based milk is not recommended in children younger than 12 months.21 Soy milk, which is fortified with calcium and protein, may be used for less than 1 week if other avenues are exhausted. Lastly, toddler formula is not interchangeable with infant formula due to its differing nutritional value.3,6 Toddler formula is intended to be supplemented with an oral diet for toddlers. These formulas should only be used for children 12 months or older for a few days if there is no other choice.6,12,21
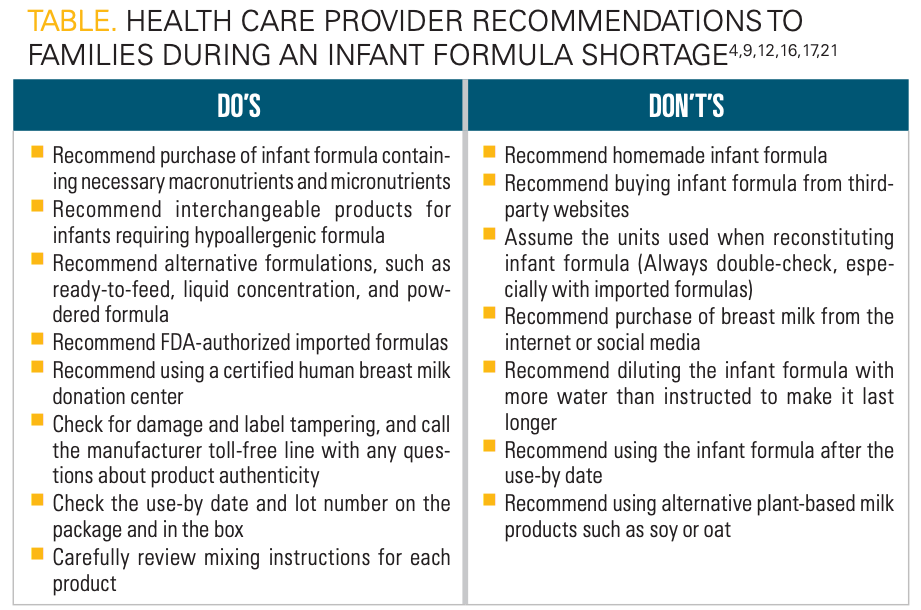
HCPs should be prepared to discourage conservation practices that could lead to unintentional infant harm. For example, in an effort to make infant formulas last longer, caregivers may dilute the product with more water. Infant formula should never be overdiluted, as it will offset the nutritional concentration and cause electrolyte disturbances.6,12,21 These complications can be fatal. In addition, homemade formulas should be discouraged, as they do not meet the nutritional or safety standards that commercial products have.21 Online recipes for homemade formulas may contain inadequate or excess amounts of vitamins and minerals and have been linked to severe, life-threatening complications.12 A case series published by the AAP described 2 patients who were fed with organic homemade infant formulas after transitioning from exclusive breastfeeding by the age of 1 month.22 The most notable laboratory abnormalities were related to inadequate vitamin D supplementation. Low levels of vitamin D resulted in inadequate calcium and phosphorus absorption and subsequent breakdown of bone. Further complications experienced by the patients included bone demineralization, cardiac arrest, hepatotoxicity, and ischemia of multiple organs. A summary of the do’s and don’ts for HCP recommendations during an infant formula shortage can be found in the Table.4,9,12,16,17,21
Click table to enlarge.
Many homemade infant formula recipes can be found online and often contain ingredients that are harmful to infant growth and development. A 2020 study analyzed 149 homemade infant formula recipes distributed over 59 online blogs.23 A total of 24.3% of the recipes used whole unpasteurized cow’s milk, 23.6% used raw goat’s milk, and 14.5% used liver as the protein base for the homemade infant formula. Pasteurization is the process where raw milk is heated at a controlled temperature to reduce pathogens.24 Unpasteurized or raw milk is associated with outbreaks of foodborne illnesses such as Salmonella and Listeria infection. Liver contains high levels of vitamin A, which can lead to vomiting and bulging of the infant’s fontanelle.25 Additionally, only 84% of recipes included instructions for proper formula storage and 18.8% included shelf-life recommendations.23 Improper storage leads to an increased risk of bacteria proliferation and subsequent infection. Approximately 75% of the blogs did not encourage pediatrician consultations prior to the usage of a homemade formula.
Conclusion
HCPs are a vital source of information during the infant formula shortage and can provide reliable and safe resources to caregivers in need. It is imperative that HCPs discourage practices that can lead to unintentional infant harm. Education should be provided to all caregivers regarding proper feeding of infants and handling of infant formula.
Click here for more from the May issue of Contemporary Pediatrics.
References:
- Facts. CDC. Updated August 7, 2023. Accessed March 9, 2024. https://www.cdc.gov/breastfeeding/data/facts.html
- Breastfeeding. World Health Organization. Accessed March 9, 2024. https://www.who.int/health-topics/breastfeeding
- Dietary Guidelines for Americans, 2020-2025. US Department of Agriculture; US Department of Health and Human Services. December 2020. Accessed March 9, 2024. https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
- Ilardi L, Proto A, Ceroni F, et al. Overview of important micronutrients supplementation in preterm infants after discharge: a call for consensus. Life (Basel). 2021;11(4):331. doi:10.3390/life11040331
- Vitamin D. CDC. Updated July 22, 2021. Accessed November 27, 2023. https://www.cdc.gov/nutrition/infantandtoddlernutrition/vitamins-minerals/vitamin-d.html
- Choosing an infant formula. CDC. Updated June 29, 2023. Accessed November 27, 2023. https://www.cdc.gov/nutrition/infantandtoddlernutrition/formula-feeding/choosing-an-infant-formula.html
- Iron. CDC. November 16, 2021. Accessed November 27, 2023. https://www.cdc.gov/nutrition/infantandtoddlernutrition/vitamins-minerals/iron.html
- Infant and young child feeding in emergencies (IYCF-E) toolkit. CDC. Updated May 12, 2023. Accessed March 12, 2024. https://www.cdc.gov/nutrition/emergencies-infant-feeding/index.html
- Information for families during the infant formula shortage. CDC. Updated July 6, 2022. Accessed March 12, 2024. https://www.cdc.gov/nutrition/infantandtoddlernutrition/formula-feeding/infant-formula-shortage.html
- FDA investigation of Cronobacter infections: powdered infant formula (February 2022). FDA. Updated August 1, 2022. Accessed December 13, 2023. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigation-cronobacter-infections-powdered-infant-formula-february-2022
- Reckitt/Mead Johnson Nutrition voluntarily recalls certain Nutramigen hypoallergenic powdered infant formula products. FDA. January 10, 2024. Accessed March 10, 2024. https://www.fda.gov/food/cfsan-constituent-updates/reckittmead-johnson-nutrition-voluntarily-recalls-certain-nutramigen-hypoallergenic-powdered-infant
- Information for families during the formula shortage. US Department of Health and Human Services. Updated July 11, 2022. Accessed December 13, 2023. https://www.hhs.gov/formula/index.html
- NASPGHAN tools for infants and children affected by formula shortages. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. August 23, 2022. Accessed December 13, 2023. https://naspghan.org/recent-news/naspghan-tools-for-hcps-affected-by-formula-recall/
- Jana L, Shu J. Forms of baby formula: powder, concentrate & ready-to-feed. healthychildren.org. Updated May 9, 2022. Accessed March 10, 2024. https://www.healthychildren.org/English/ages-stages/baby/formula-feeding/Pages/Forms-of-Baby-Formula.aspx
- Fuchs GJ III. Is it safe to buy imported baby formulas online? healthychildren.org. May 11, 2022. Accessed March 12, 2024. https://www.healthychildren.org/English/tips-tools/ask-the-pediatrician/Pages/Is-it-OK-to-buy-imported-formulas-online.aspx
- Enforcement discretion to manufacturers to increase infant formula supplies. FDA. Updated February 27, 2023. Accessed March 10, 2024. https://www.fda.gov/food/infant-formula-guidance-documents-regulatory-information/enforcement-discretion-manufacturers-increase-infant-formula-supplies
- Questions & answers for consumers concerning infant formula. FDA. Updated May 17, 2023. Accessed March 10, 2024. https://www.fda.gov/food/people-risk-foodborne-illness/questions-answers-consumers-concerning-infant-formula
- Human Milk Banking Association of North America. Accessed March 10, 2024. https://www.hmbana.org/welcome2.html
- Infant food and feeding. American Academy of Pediatrics. Updated November 28, 2023. Accessed December 13, 2023. https://www.aap.org/en/patient-care/healthy-active-living-for-families/infant-food-and-feeding/
- Leung AK, Sauve RS. Whole cow’s milk in infancy. Paediatr Child Health. 2003;8(7):419-421. doi:10.1093/pch/8.7.419
- Abrams SA. Baby formula shortages: what parents need to know. American Academy of Pediatrics. May 16, 2022. Accessed December 15, 2023. https://publications.aap.org/DocumentLibrary/Solutions/PPE/BabyFormulaShortages_ppe_document263_en.pdf
- Vieira MA, Kube PK, van Helmond JL, et al. Recipe for disaster: homemade formula leading to severe complications in 2 infants. Pediatrics. 2021;148(3):e2021050947. doi:10.1542/peds.2021-050947
- Davis SA, Knol LL, Crowe-White KM, Turner LW, McKinley E. Homemade infant formula recipes may contain harmful ingredients: a quantitative content analysis of blogs. Public Health Nutr. 2020;23(8):1334-1339. doi:10.1017/S136898001900421X
- Raw milk questions and answers. CDC. Updated November 1, 2023. Accessed March 12, 2024. https://www.cdc.gov/foodsafety/rawmilk/raw-milk-questions-and-answers.html
- Mahoney CP, Margolis MT, Knauss TA, Labbe RF. Chronic vitamin A intoxication in infants fed chicken liver. Pediatrics. 1980;65(5):893-897.
