Multisystem inflammatory syndrome in children associated with COVID-19
Children who at first did not appear to contract COVID-19 have been found to exhibit a more serious illness that has now been reported worldwide. Two experts offer guidance on recognizing and treating multisystem inflammatory syndrome in children (MIS-C).
Since the first report of an outbreak of respiratory disease in Wuhan, China, in December 2019 caused by a novel coronavirus (SARS CoV2), coronavirus disease (COVID-19) has reached pandemic proportions. As of June 6, 2020,1,2 there were 6,663,304 cases and 392,802 deaths reported worldwide, with 1,891,690 cases and 109,192 deaths reported in the United States. Children and infants have been relatively spared from severe COVID-19, with pediatric cases accounting for less than 2% of COVID hospitalizations.
However, in late April, reports began to emerge of a multisystem inflammatory syndrome in children (MIS-C) related to SARS-CoV-2 infection. The Centers for Disease Control and Prevention (CDC) established the following definition for MIS-C:3
- An individual aged 21 years and younger with fever for 1 or more days, laboratory evidence of inflammation, and illness requiring hospitalization with multisystem (2 or more) organ involvement (cardiac, renal, hematologic, gastrointestinal, dermatologic, neurologic), AND
- No alternative plausible diagnosis, AND
- Evidence of current or recent SARS-CoV-2 infection by reverse transcription polymerase chain reaction (RT-PCR), serology or antigen test, or exposure to a suspected or confirmed COVID-19 case within the 4 weeks prior to the onset of symptoms.
This definition provides a starting point for addressing this newly described hyperinflammatory syndrome with COVID-19, but it is also overly broad without adequately defining the nature of the multisystem involvement and only requiring 1 or more days of fever. The very elevated inflammatory (C-reactive protein [CRP], ferritin, triglycerides) and myocardial markers (N-terminal-pro B-type natriuretic peptide [NT-proBNP] and troponin) and associated thrombocytopenia and lymphopenia do appear to be useful in consideration of a child with possible MIS-C.
MIS-C case series
Initial series of cases compatible with the diagnosis of MIS-C have been published from Europe. Riphagen and colleagues described 8 previously healthy children aged 4 to 14 years from Evelina London Children’s Hospital who presented with hyperinflammatory shock and features similar to atypical Kawasaki disease (KD), KD shock syndrome (KDSS), or toxic shock syndrome (TSS) in mid-April 2020.4 Of the 8 cases, 6 were children of African-Caribbean descent, most were teenagers, and 5 were males. All tested negative for SARS-COV2 by PCR but all had detectable antibodies to the virus.
Prominent clinical features in these cases included high fevers, gastrointestinal symptoms, and shock requiring mechanical ventilation in 7 of the cases, and a lack of respiratory symptoms. Laboratory parameters were notable for elevated CRP, procalcitonin, ferritin, D-dimers, triglycerides, troponin, and NT-proBNP. All were treated with intravenous immunoglobulin (IVIG), pressors, and antibiotics. Six received aspirin. One death occurred in a child with refractory shock and an arrhythmia requiring extracorporeal membrane oxygenation (ECMO) who developed a cerebrovascular infarct. One child developed a giant coronary aneurysm. The others were discharged from intensive care in 4 to 6 days and recovered.
A series of 10 children aged 3 to 16 years (mostly teenagers) from Bergamo, Italy, presenting between February 18 and April 20, 2020, with what the authors labeled as severe Kawasaki-like disease was published in the Lancet on May 13, 2020.5 By definition, these children met the 2017 American Heart Association criteria for KD or incomplete KD with 5 or more days of fever, compatible rash, and mucous membrane findings, but the overall picture was not consistent with classic KD.
Additionally, diarrhea was reported at presentation in 6 of the 10 children. Two of the children were positive for SARS-CoV2 by PCR; 8 were positive for SARS-CoV2 antibodies; and 2 were negative by PCR and serologic testing. Laboratory testing was notable for increased inflammatory markers, lymphopenia and thrombocytopenia (8/10 for each), increased triglycerides, increased ferritin, elevated proBNP (10/10), and troponin (5/9 tested). Five of the 10 presented in shock and 5 met criteria for macrophage activation syndrome (MAS). All received IVIG and aspirin; 8 of 10 were administered adjunctive steroids. All the children recovered and continue to be followed for development of coronary aneurysms.
More recently, a large French series of 35 patients with MIS-C was recently published in Circulation.6 These previously healthy children were cared for at 1 Swiss and 12 French hospitals from March 22 to April 30, 2020, utilizing the criteria of fever, cardiogenic shock or left ventricular ejection fraction (LVEF) less than 50%, and elevated CRP. The median age was 10 years with only 1 of 35 aged younger than 5 years and 19 of 35 aged from 11 to 16 years. SARS-CoV-2 testing in this group was positive in 31 of 35, with RT-PCR positive in 14 of 35 (12 nasopharyngeal and 2 fecal) and positive serology in 30 of 35 including IgG positive in 28. In 13 of 35 patients there was a recent family member with viral symptoms.
The clinical features of these 35 patients included fever and asthenia in all; 83% had abdominal pain associated with diarrhea and/or vomiting; 65% had respiratory distress; and some had rash or lip inflammation. None met the diagnostic criteria for KD. Twenty-nine of 35 were admitted to the pediatric intensive care unit (PICU) with cardiogenic shock, two-thirds were mechanically ventilated, and 10 of 35 received circulatory assistance with venous-arterial ECMO.
Echocardiographic features included 10 of 35 children with LVEF less than 30% and 25 of 35 with LVEF between less than 30% to 50%. Global left ventricular hypokinesis was present in 31 of 35 and segmental left ventricular hypokinesis in 3 more. The evolution of LVEF impressively improved rather quickly, with mean (and SD) LVEF equal to 32% (9%) at baseline in all 35, improved on day 3 to 52% (10%) in 23, and on day 7 to 60% (6%) in 34 patients. Six of 35 patients had a coronary artery z score greater than 2.0, although 5 of these 6 involved the left main coronary artery (of little significance per recent guidelines) and 1 involved the right coronary artery. No coronary aneurysms were detected.
Laboratory features of these patients were very striking and included median CRP of 24 mg/dL (range 15-31); high-sensitivity (hs) troponin of 347 ng/mL (range 186-1267; normal, <26 ng/mL); NT-proBNP, 41,000 pg/mL (range, 35,000-52,000; normal, <300 pg/mL); D-dimers, 5284 ng/mL (range 4000-9000; normal, <500 ng/mL); and interleukin (IL)-6, 135 pg/mL (range 87-175; normal, <8.5 pg/mL).
Treatment of the 35 patients included 28 who received inotrope(s), 25 treated with IVIG, 12 who received IV steroids (as in patients with high-risk KD), 3 received anakinra, and 25 of 35 were treated with heparin. The outcomes of the 35 children are remarkable in that all survived after a median 7-day PICU stay and 10-day total hospital stay. Twenty-five of 35 were at home with LVEF greater than 60% at day 7 with an additional 3 children at home with LVEF greater than 45%. LV function normalized in 25 of 35 patients at a mean of 2.2 days. No patients experienced thromboses or emboli.
A second similar French series of 21 patients included 12 (57%) who had at least 1 parent from sub-Saharan Africa or a Caribbean island.7
In the United States, the majority of cases of MIS-C have been reported from New York. The New York State Department of Health has reported 202 cases of MIS-C with 3 deaths from late April 2020 through June 6, 2020.8 Blacks are overrepresented, accounting for 31% of the cases compared with 22% for white cases, 17% other, 26% unknown, and 3% Asian. The median age of these children is about 9 years.
Comparison of MIS-C with KD
Whereas MIS-C patients may present with one or more features reminiscent of KD, there are many other, often strikingly different features of the 2 conditions, supporting the concept that they are fundamentally different illnesses (Table). The study from Italy5 by definition selected children with KD-like features, yet many differences in these cases from classic KD are apparent.
Table
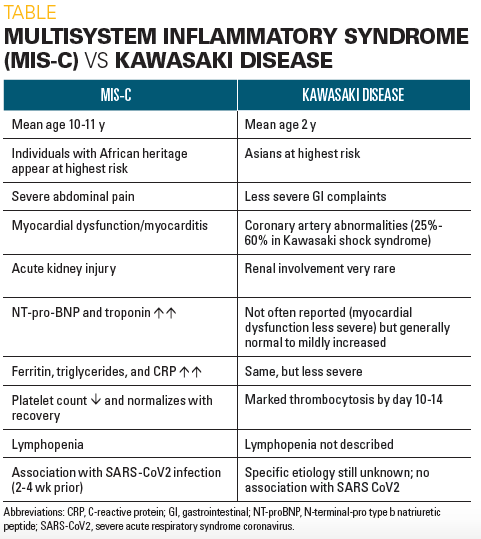
There are markedly different demographic features in these 2 conditions, with very distinctive age and racial distributions. Whereas the median age of KD children is 2 years and 80% are aged younger than 5 years, MIS-C patients are much older with a median age of 10 to 11 years in most series and include many adolescents, teenagers, and even a few young adults. Additionally, the attack rates for KD are very much higher among Japanese, Korean, and Chinese children compared with other racial groups whereas Asian populations have been very underrepresented in MIS-C reports to date despite marked SARS-2 exposures. Several of the series of MIS-C patients strongly suggest overrepresentation of children of African backgrounds.
In memoriam
Although some of the dermatologic and mucosal features of KD may be present, MIS-C patients typically present with much more severe abdominal complaints of pain with diarrhea and/or vomiting compared with KD patients, as well as manifesting multiorgan system involvement and cardiogenic shock. A small (approximately 2%) subset of KD patients can develop KDSS but they often have significant coronary abnormalities as well. The latter patients are somewhat clinically similar to those with TSS.
Additionally, the hallmark of KD is the development of coronary artery abnormalities, whereas evidence of significant myocardial involvement is mainly limited to the small group of KDSS patients. In striking contrast, MIS-C patients typically present with shock and with moderate-to-severe myocardial dysfunction with low LVEFs, and only a relative handful have had coronary abnormalities, often mild and of unclear significance.
By definition, MIS-C patients have evidence of recent SARS-CoV-2 infection, while KD patients do not. Some of the laboratory features of MIS-C strongly resemble those of MAS such as elevated ferritin, D-dimers, triglycerides, and some are indicative of myocardial dysfunction such as elevated troponin and very elevated NT-proBNP-levels in the greater-than-30,000 pg/mL range. In addition, MIS-C patients typically have lymphopenia, neutrophilia, thrombocytopenia, and very high CRP levels on presentation, unlike KD patients who rarely share these abnormalities and have more modestly elevated CRP levels.
MIS-C webinar

Overall, the picture of MIS-C in many respects closely resembles the later phase seen in many adult COVID-19 patients of “cytokine storm,” with hyperinflammation, multiorgan damage including kidney and myocardium, with features of MAS and TSS. It is not at all clear why MIS-C patients have almost always had no early COVID-19 manifestations and that their illnesses typically present 4 to 6 weeks after the peak COVID-19 activity in a region. In this regard, MISC can be considered a postinfectious illness. Of great interest is the fact that MIS-C patients typically respond very well to IVIG with or without IV steroid, probably by effectively modulating cytokine activation. The role of additional therapies including anticoagulation with low-dose molecular heparin, aspirin, or the IL-1 inhibitor anikinra remains speculative at present.
In conclusion
In summary, the newly described MIS-C is quite distinct from KD. It is likely a postinfectious inflammatory syndrome as evidenced by the delay in onset from the peak of COVID cases, by the time from the apparent exposure to SARS-CoV-2 to onset of symptoms in many of the reported cases, and by the very marked elevations in inflammatory and myocardial markers observed in these cases. Thus MIS-C may be analogous to the later phase of adult COVID-19 that is also characterized by cytokine storm that may include severe myocardial dysfunction, acute kidney injury, and laboratory markers of MAS.
References
- World Health Organization (WHO). Coronavirus disease (COVID-19). Situation report—138. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200606-covid-19-sitrep-138.pdf?sfvrsn=c8abfb17_4. Published June 6, 2020. Accessed June 12, 2020.
- Centers for Disease Control and Prevention (CDC). Coronavirus disease 2019 (COVID-19). Cases in the US. Available at: (www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Updated June 11, 2020. Accessed June 12, 2020.
- Centers for Disease Control and Prevention (CDC). Multisystem inflammatory syndrome (MIS-C). Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). Case definition for MIS-C. Available at: https://www.cdc.gov/mis-c/hcp/ . Reviewed May 29. 2020. Accessed June 12, 2020.
- Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. May 7, 2020. Epub ahead of print. doi: 10.1016/S0140-6736(20)31094-1
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. May 13, 2020. Epub ahead of print. doi: 10.1016/S0140-6736(20)31103-X
- Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. May 17, 2020. Epub ahead of print. doi: 10.1161/CIRCULATIONAHA.120.048360
- Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136bmj.m2094
- New York State. Novel coronavirus. Childhood inflammatory disease related to COVID-19. Available at: https://coronavirus.health.ny.gov/childhood-inflammatory-disease-related-covid-19. Updated June 12, 2020. Accessed June 12, 2020.

Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.