Pediatric Musculoskeletal Infections: Managing the Significant Organisms
Musculoskeletal infections in children include osteomyelitis, septic arthritis, and pyomyositis. Most of these infections are bacterial.
Musculoskeletal infections in children include osteomyelitis, septic arthritis, and pyomyositis. Most of these infections are bacterial. Staphylococcus aureus is the most common organism in children in all age categories. Others include group A Streptococcus, Neisseria meningitidis in purpura fulminans, Streptococcus pneumoniae, Neisseria gonorrhoeae, Mycobacterium tuberculosis, and Borrelia burgdorferi. In this article, we describe the significant organisms to watch out for in pediatric musculoskeletal infections.
GROUP A STREPTOCOCCUS
This organism is responsible for several types of musculoskeletal infections in children, especially septic arthritis. Of note, the most common complication of chickenpox is group A Streptococcus superinfection of the varicella lesions. Usually, it has been the infecting organism in reported cases of septic arthritis. In one study, Streptococcus pyogenes was reported to cause septic arthritis in chickenpox, especially in cases without bacterial superinfection of the skin.1
The most serious complication involves a toxic shock–like syndrome characterized by severe local tissue destruction and life-threatening systemic manifestations.2 When group A Streptococcus infection is associated with fulminant findings, aggressive resuscitation is necessary, along with timely surgical decompression of the foci of infection after a vigilant search for such sites. S pyogenes also may cause osteomyelitis or septic arthritis by hematogenous inoculation.3
METHICILLIN-RESISTANT S AUREUS (MRSA)
Musculoskeletal infection caused by MRSA is common in septic arthritis and osteomyelitis. Hospital-acquired MRSA (HA-MRSA) may arise primarily in premature infants in neonatal ICUs. During the past 7 years, however, community-associated MRSA (CA-MRSA) has emerged as a major pathogen in many areas of the United States and around the world.4,5 Now it is emerging as a major cause of skin and soft tissue infections in the sports community.
Transmission of HA-MRSA is observed in patients who are admitted to burn units or ICUs, undergo surgery, are hospitalized for prolonged periods, undergo invasive procedures, or are exposed to patients colonized with MRSA.6 CA-MRSA is observed in children who were treated with an antibiotic in the preceding year, have compromised skin integrity, live in crowded conditions, or participate in team sports7; this type differs from HA-MRSA in disease presentation and epidemiology, and it can be a very aggressive disease. At least part of the pathogenicity of CA-MRSA can be explained by production of Panton-Valentine leukocidin, a cytotoxin that causes white blood cell destruction and severe soft tissue necrosis.
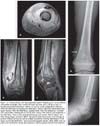
If CA-MRSA is suspected (Figure), the first-line options include administration of clindamycin and then the addition of vancomycin in critically ill patients.8 Vancomycin is the first-line agent for HA-MRSA infection. Clindamycin may be given as the first-line drug for CA-MRSA infection, particularly in patients who are allergic to or intolerant of b-lactam antibiotics. Although a number of the newer antibiotics (eg, daptomycin, dalbavancin, tigecycline, ceftobiprole, linezolid, and quinopristin/dalfopristin) have been shown to be effective against MRSA, these agents should be used only with the advice of an infection specialist.
In most patients, the clinical response determines the duration of therapy. The number of febrile days and hospital days has increased in children with musculoskeletal infection caused by MRSA.9
Vander Have and associates7 described 27 children with musculoskeletal infection caused by CA-MRSA. Twelve patients required admission to an ICU; multisystem failure developed in 4 of them. Seven patients showed evidence of deep venous thrombosis or septic pulmonary emboli. All patients required surgical debridement. The authors concluded that surgical debridement and long-term antibiotic therapy are necessary to manage this life-threatening infection.
N MENINGITIDIS IN PURPURA FULMINANS
Although N meningitidis usually is not associated with direct infection of musculoskeletal structures, it is significant as a causal organism in purpura fulminans that produces devastating musculoskeletal consequences. This is characterized by the acute onset of progressive dermal vascular thrombosis, disseminated intravascular coagulation, and shock.10 Treatment involves initial resuscitation with appropriate antibiotics. A third-generation cephalosporin, such as ceftriaxone, usually is effective.
S PNEUMONIAE
This organism is more commonly associated with bacteremia, pneumonia, or meningitis but has been reported as a causative organism for osteomyelitis and septic arthritis in infants and children. The femur and humerus are the bones most often affected, and the hip and knee are the joints most commonly involved. Immaturity of the child and concomitant immunodeficiencies have been reported to increase the risks of S pneumoniae infection.11
N GONORRHOEAE
Septic arthritis caused by N gonorrhoeae is monarticular or pauciarticular. Gonococcal bacteremia is more likely to be associated with polyarthralgias and skin lesions.
When gonococcal infection is suspected, cultures should be obtained from joint fluid; the cervix of postpubertal girls; urethral or prostatic discharge of boys; and the vagina, pharynx, or rectum in children in whom sexual abuse is suspected. The diagnosis of N gonorrhoeae infection is confirmed by culture that needs to be done under warm conditions with low carbon dioxide levels using Thayer-Martin agar.
Management of gonococcal arthritis initially involves intravenous administration of a third-generation cephalosporin. Then, because of the prevalence of resistant strains, there is local aspiration and irrigation of the joint.
M TUBERCULOSIS
Tuberculosis infections have been on the rise in the United States, mainly because of an influx of immigrants from high-prevalence countries and marginalized populations.12 Signs and symptoms of osteomyelitis, dactylitis, or septic arthritis may take months to years to manifest. More than half of cases of tubercular osteomyelitis involve the spine, followed in incidence by infections around the hip and knee. Spinal involvement usually occurs in the anterior third of the vertebral body. Paravertebral abscess is almost pathognomonic.

Diagnosis requires a high index of suspicion, skin tests for tuberculosis using purified protein derivative, and identification of the organism in culture material. Often, the white blood cell count is normal but the erythrocyte sedimentation rate is elevated.
Current treatment recommendations involve quadruple-drug therapy (isoniazid, rifampin, pyrazinamide, and streptomycin) for 2 months, followed by isoniazid and rifampin for another 10 months. Surgical decompression is rarely needed. Indications for spinal surgery include neurological involvement, spinal instability, and failure of medical treatment.13
B BURGDORFERI
Lyme disease is caused by this spirochete; its common vector is the deer tick. Lyme disease often presents as an episodic musculoskeletal synovitis affecting 1 or more joints. It may progress to septic arthritis of the joint.
The serological tests conducted for diagnosis are the enzyme-linked immunosorbent assay and the immunoblot (Western blot) analysis. These tests should be considered only in patients in whom there is a reasonable clinical suspicion of infection. Initial treatment consists of 10 to 30 days of oral antibiotics (amoxicillin or doxycycline). In cases of neurological or cardiac manifestations or recurrence after oral therapy, intravenous ceftriaxone may be needed for 14 to 28 days.
A post–Lyme disease syndrome has been described as involving recurrent arthralgias, myalgias, headache, neck pain, and fatigue. Treatment with antibiotics is controversial, and spontaneous resolution within 6 months is common.14
References:
References:
1. Bradley TM, Dormans JP. Streptococcus pyogenes septic arthritis of the elbow complicating the chicken pox. Pediatr Emerg Care. 1997;13:380-381.
2. Jackson MA, Burry VF, Olson LC. Multisystem group A beta-hemolytic streptococcal disease in children. Rev Infect Dis. 1991;13:783-788.
3. Perlman MH, Patzakis MJ, Kumar PJ, Holtom P. The incidence of joint involvement with adjacent
osteomyelitis in pediatric patients. J Pediatr Orthop. 2000;20:40-43.
4. Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178-182.
5. Gwynne-Jones DP, Stott NS. Community-acquired methicillin-resistant Staphylococcus aureus: a cause of musculoskeletal sepsis in children. J Pediatr Orthop. 1999;19:413-416.
6. Layton MC, Hierholzer WJ Jr, Patterson JE. The evolving epidemiology of methicillin-resistant Staphylococcus aureus at a university hospital. Infect
Control Hosp Epidemiol. 1995;16:12-17.
7. Vander Have KL, Karmazyn B, Verma M, et al. Community-associated methicillin-resistant Staphylococcus aureus in acute musculoskeletal infection in children: a game changer. J Pediatr Orthop. 2009;29:927-931.
8. Steer AC, Carapetis JR. Acute hematogenous osteomyelitis in children: recognition and management. Paediatr Drugs. 2004;6:333-346.
9. Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, et al. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23:701-706.
10. White B, Livingstone W, Murphy C, et al. An open-label study of the role of adjuvant hemostatic support with protein C replacement therapy in purpura fulminansâassociated meningococcemia. Blood. 2000;96:3719-3724.
11. Bradley JS, Kaplan SL, Tan TQ, et al. Pediatric pneumococcal bone and joint infections. The Pediatric Multicenter Pneumococcal Surveillance Study Group (PMPSSG). Pediatrics. 1998;102:1376-1382.
12. Furin J, Johnson JL. Recent advances in the diagnosis and management of tuberculosis. Curr Opin Pulm Med. 2005;11:189-194.
13. Vohra R, Kang HS, Dogra S, et al. Tuberculous osteomyelitis. J Bone Joint Surg. 1997;79B:562-566.
14. Weinstein A, Britchkov M. Lyme arthritis and postâLyme disease syndrome. Curr Opin Rheumatol. 2002;14:383-387.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.