Recommended Vitamin D Intake in Children: Reasons for the Recent Increase
The American Academy of Pediatrics (AAP) recently doubled the amount of vitamin D that it recommends all infants, children, and adolescents receive each day-from 200 to 400 IU. Also new is the academy's recommendation that vitamin D supplementation begin as soon after birth as possible. Supplementation is recommended in infants who do not receive 400 IU per day from formula.
ABSTRACT: The American Academy of Pediatrics (AAP) recently doubled the amount of vitamin D that it recommends all infants, children, and adolescents receive each day-from 200 to 400 IU. Also new is the academy's recommendation that vitamin D supplementation begin as soon after birth as possible. Supplementation is recommended in infants who do not receive 400 IU per day from formula. Although there is growing evidence that vitamin D deficiency is associated with a wide range of chronic nonskeletal diseases, it has yet to be proved that vitamin D levels play a causal role in the development of these conditions. Thus, the chief basis for the increase in the AAP's recommended daily intake was evidence that this is the amount of the vitamin needed to prevent rickets in young children.
In recent years, evidence has mounted suggesting that vitamin D is more important to human health than had previously been realized. In light of this growing body of evidence, the American Academy of Pediatrics (AAP), in October 2008, officially recommended increasing the vitamin D intake of all infants, children, and adolescents from 200 to 400 IU per day.1
In this article, I review new information on the role vitamin D plays in health, and I explain the reasoning behind the revised AAP recommended vitamin D intake. I also offer practical guidance on determining which children should receive supplementation to ensure that their intake meets the new standard.
THE "NEW" IMPORTANCE OF VITAMIN D
It is becoming increasingly clear that vitamin D plays a vital role in children's health. It has long been known that vitamin D deficiency can cause rickets. What may be less well known is that rickets is still seen in American infants and, in fact, is not all that rare. Vitamin D deficiency rickets in the United States occurs most commonly in infants between the ages of 6 and 18 months; it is rarely reported in children older than 5 years.2-4 There is no required reporting of this diagnosis, and it is estimated that fewer than half of the children with rickets are even hospitalized.5
Most cases of rickets reported in the United States in the past 15 years have occurred in exclusively breast-fed, African American infants.5-8 These cases have paralleled the major efforts of Women, Infants, and Children (WIC) clinics throughout the country to increase the rate of exclusive breast-feeding in this population. Unfortunately, these government-funded clinics cannot dispense dietary supplements. The result is that exclusive breast-feeding is being encouraged in the very population that is at most risk for vitamin D deficiency rickets. At present, only 3 states (North Carolina, Alaska, and Maryland) have put into effect plans to supplement these high-risk infants with vitamin D.7,9,10
The importance of vitamin D may go far beyond the prevention of rickets, however. Vitamin D deficiency has been associated with many chronic diseases. These include hypertension, atherosclerosis/myocardial infarction, type 1 and type 2 diabetes, colorectal cancer, prostate cancer, breast cancer, ovarian cancer, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and even wheezing in early childhood.1,11
A VITAMIN THAT ALSO FUNCTIONS AS A HORMONE
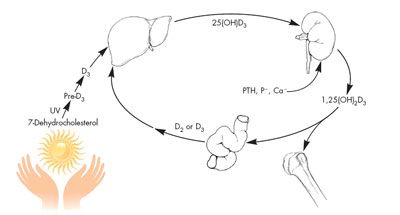
One reason that vitamin D may play a role in such a wide array of conditions is that vitamin D functions metabolically as a hormone, not as a vitamin. Like all hormones in the steroid family, its synthesis begins with cholesterol (Figure 1). With exposure to the UVB radiation in sunlight, 7-dehydrocholesterol in the skin is converted to the parent compound, vitamin D3 (cholecalciferol). Cholecalciferol is then hydroxylated in the liver to form 25-hydroxyvitamin D3 (25[OH] vitamin D3, or calcidiol) and hydroxylated again in the kidney to form 1,25-dihydroxyvitamin D3 (1,25[OH]2 vitamin D3, or calcitriol). 1,25(OH)2 vitamin D3 then acts in concert with parathyroid hormone to tightly regulate calcium metabolism, primarily by controlling the intestinal absorption of calcium.12 1,25(OH)2 vitamin D3 is a true hormone that effects gene transcription at the cellular level. It does this through its interaction with a genomic (nuclear) vitamin D receptor that is present in many types of cells.13 Recently, a second receptor for 1,25(OH)2 vitamin D3 has been described on the plasma membrane of many cells.14
DETERMINING ADEQUATE VITAMIN D STATUS
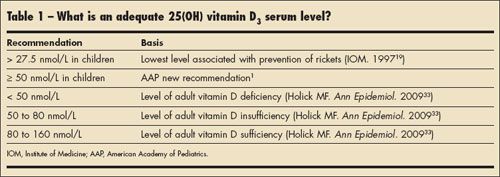
Although 1,25(OH)2 vitamin D3 is the true hormone, the most plentiful form of the vitamin in circulation (by a factor of 10) is 25(OH) vitamin D3. Thus, the 25(OH) vitamin D3 level is thought to be the best marker of a patient's vitamin D status. What constitutes an adequate level of 25(OH) vitamin D3 has been the subject of much debate in recent years. Recent thinking on this question is summarized in Table 1. Keep in mind that to determine "adequate" biological levels of 25(OH) vitamin D3 in both adults and children, functional outcomes associated with these levels must be documented.
Can risk of chronic nonskeletal diseases determine adequate vitamin D levels? Although there is evidence of an association between vitamin D deficiency and a number of chronic diseases, there are no adequate clinical trials demonstrating that vitamin D is causally related to these disease processes. Thus, such outcomes cannot be used to establish adequate levels of 25(OH) vitamin D3.
One of the most frequently cited studies in the literature on vitamin D in pediatrics is a Finnish study showing a relationship between the incidence of type 1 diabetes and vitamin D intake during childhood.15 This study was done in Lapland, whose population has the highest known incidence of type 1 diabetes. That vitamin D may play a role in type 1 diabetes is supported by the observation that the disease is far more common in northern latitudes where sunshine exposure is decreased and serum 25(OH) vitamin D3 levels are low in winter. There are other reasons supporting such a connection as well. Vitamin D appears to stimulate insulin release and insulin receptor expression in animal and cell culture models; type 1 diabetes is a state of chronic inflammation,13 and vitamin D is known to have anti-inflammatory effects. Finally, vitamin D may play a role in immune function, and type 1 diabetes is known to be an autoimmune process directed against the beta cells of the pancreas. Nonetheless, the Finnish study has several weaknesses. It was a retrospective birth cohort study in children born in 1966 during a time when the recommended vitamin D intake for children in this part of Finland was 2000 IU per day. Although serum 25(OH) vitamin D3 levels are known to be low in northern Finland, no 25(OH) vitamin D3 levels were measured in the study's participants, and their vitamin D intake was estimated from retrospective chart reviews of medical records. Thus, although the epidemiological association appears to be strong, this study does not shed light on the question of whether vitamin D plays a causative role in type 1 diabetes, a disease with a very strong genetic component.
A relationship between bone health outcomes and vitamin D levels? Potential functional outcomes in infants and children that might be used to establish adequate levels of 25(OH) vitamin D3 include the presence or absence of vitamin D deficiency rickets, the presence or absence of a negative correlation with serum parathyroid hormone (PTH) levels, degree of bone mineralization (as determined by medical imaging), amount of calcium absorption, and number of bone fractures. There is clearly not enough data on the relationship between 25(OH) vitamin D3 levels and calcium absorption or fracture rates in children to use these as functional outcomes.
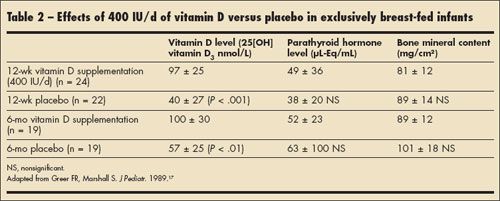
Regarding 25(OH) vitamin D3 levels and measures of bone mineral content, there are only 2 randomized controlled trials reported in the literature.16,17 Both of these trials were conducted with white, exclusively breast-fed infants who were randomly assigned to receive either 400 IU of vitamin D or placebo each day. The results of one of these trials are summarized in Table 2. In this study, serum 25(OH) vitamin D3 levels in the exclusively breast-fed infants who received 400 IU of vitamin D daily were well above 50 ng/mL and were significantly higher than levels in those infants who did not receive supplemental vitamin D. However, there was no difference in bone mineral content between the vitamin D and placebo groups (as measured by single photon absorptiometry in the left radius). This study also showed that there was no relationship between vitamin D intake and PTH measurements in the 2 groups of infants. Thus, data indicating that bone mineral or serum PTH level in infants is related to 25(OH) vitamin D3 level are lacking at this time. In adolescents, who have the lowest 25(OH) vitamin D3 levels in the US population, there is some evidence that PTH levels and serum 25(OH) vitamin D3 levels can be used as functional outcomes for vitamin D adequacy, with a weak correlation between the two (r = 0.29; P < .001) in at least 1 study.18
Absence of rickets the best outcome for determining adequate vitamin D status in children. One would naturally assume that there would be a serum level of 25(OH) vitamin D3 above which rickets never occurred. In fact, the Institute of Medicine (IOM) report of 1997 set the lower limit of acceptable serum 25(OH) vitamin D3 levels at 27.5 nmol/L, largely based on the absence of rickets when levels were above this value in the People's Republic of China-with lesser amounts of data from the United States and Norway used.19
However, if one looks at the reports of rickets and 25(OH) vitamin D3 levels in children around the world, evidence to support an absolute threshold level of 25(OH) vitamin D3 for the occurrence of rickets is not supported.20 There are 5 studies in which all the cases of rickets were associated with serum 25(OH) vitamin D3 levels of less than 27.5 nmol/L; however, in 6 other studies, cases of rickets occurred in children with serum levels between 30 and 50 nmol/L.20
Although many of these studies are from developing countries and are perhaps confounded by calcium intake (severe calcium deficiency is also associated with rickets) and lack of international standards for measuring levels of 25(OH) vitamin D3, at least 2 studies are from the United States.21,22 In one study involving 43 cases of nutritional rickets, the mean serum 25(OH) vitamin D3 level was 52.2 ± 28.7 nmol/L (range, 11.7 to 137 nmol/L) at the time of diagnosis. Of the 43 children in the study, 34 (86%) were African American, with a mean age at presentation of 20 months; 93% of the infants had been breast-fed for an average of 13.2 ± 5.5 months. Fifteen percent of the infants had received some vitamin D supplements, and most had been weaned to diets that contained minimal amounts of dairy products.22 Thus, clearly, cases of rickets with serum 25(OH) vitamin D3 levels above 27.5 nmol/L have been reported even in the United States.
One can therefore conclude that there is good evidence that rickets can develop in the first 6 months of life in unsupplemented breast-fed infants who have 25(OH) vitamin D3 levels of 50 nmol/L or even higher. The AAP has set 50 nmol/L as the cutoff for acceptable 25(OH) vitamin D3 levels in children. This decision was largely based on the evidence in infants and young children that 400 IU of vitamin D per day will maintain serum 25(OH) vitamin D3 levels above 50 nmol/L-and that infants receiving such supplementation do not get rickets. At present, the AAP does not recommend routine screening of 25(OH) vitamin D3 levels in otherwise healthy children. Such a practice cannot be justified given the current knowledge base.
WHAT IS THE VITAMIN D STATUS OF AMERICAN CHILDREN?
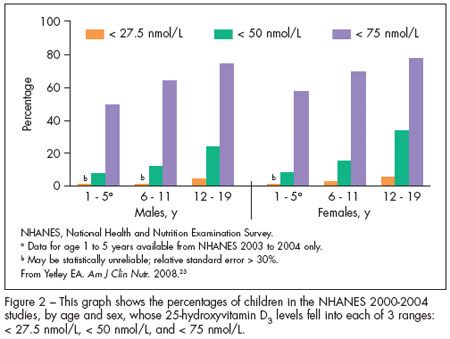
The National Health and Nutrition Examination Survey (NHANES) from 2000 to 2004 used serum measurements of 25(OH) vitamin D3 to determine the vitamin D status of the US population by age, sex, race/ethnicity, and pregnancy status.23 The surveys included data on 895 children between the ages of 1 and 5 years and 1837 children between 6 and 11 years. The mean serum level for children between 1 and 5 years of age was 74 nmol/L and the mean serum level for children between 6 and 11 years was 68 nmol/L, well above the 50 nmol/L cutoff for acceptable 25(OH) vitamin D3 levels set by the AAP. An overview of the data from this survey is shown in Figure 2, with the data for both males and females in each age-group presented. The percentage of children and adolescents with serum 25(OH) vitamin D3 levels of less than 27.5 nmol/L was extremely small in these surveys. However, a significant number of adolescents (aged 12 to 19 years) had serum levels of less than 50 nmol/L.
Another report, from the NHANES data of 1999 to 2000, found that 34% of children and adolescents (aged 2 to 17 years) took vitamin supplements that would have contained at least 400 IU of vitamin D.24 However, this study also showed that the children most likely to benefit from these supplements (those with poor nutrition and activity patterns, obesity, lower income, and restricted health care access) used vitamin supplements the least.
WHY INCREASE CHILDREN'S DAILY INTAKE OF VITAMIN D TO 400 IU?
As I noted at the beginning of this article, there was growing concern that the 1997 IOM19 recommended intake of 200 IU of vitamin D for infants and children (affirmed by the AAP1 in 2003) was not adequate. Numerous groups called on the IOM to update its 1997 recommended intake for vitamin D.25,26 How was the 400-IU amount arrived at? First, there is considerable precedent for a supplement of 400 IU of vitamin D per day in children. This is the amount contained in a teaspoon of cod liver oil.19 Also, a daily intake of 400 IU will keep 25(OH) vitamin D3 levels in formula-fed infants and breast-fed infants well above 50 nmol/L-and generally greater than 75 nmol/L-during the first year of life.16,17
In addition, it is well known that 400 IU of vitamin D per day prevents rickets in white infants, and if you give 400 IU per day long enough, it will treat rickets as well.27 Furthermore, giving any more than this does not improve the benefit of rickets prevention. Additional data are needed to extend these observations to African American infants.
Another factor in the decision to recommend increasing the daily intake of vitamin D to 400 IU-rather than the higher amounts some authorities have proposed-is that the safety of this dosage is well established. There is no evidence that 400 IU of vitamin D in children is unsafe or associated with hypervitaminosis or hypercalcemia. The IOM report set the tolerable upper intake level at 1000 IU per day in infants and 2000 IU per day in older children and adults.19 Intakes of vitamin D that exceed 1000 IU per day have never been studied for their potentially adverse effects in infants and children.
SOURCES OF VITAMIN D
Dietary sources. In the typical diet, there are few sources of vitamin D other than fatty fish. In fact, the fortification of milk and infant formulas with 400 IU/L of vitamin D is the most important dietary source for infants, children, and adolescents.12 Breast milk is very low in vitamin D (22 IU/L) and thus a poor dietary source.12 Unfortunately, it has been observed that the decreasing intake of milk in American children has resulted in reduced vitamin D intake. Other investigators have also noted that the vitamin D content of dairy products is quite variable and unpredictable, even in instances in which it is required to be 400 IU/L.28
Sunlight. Another source of vitamin D is exposure of the skin to UVB light (see Figure 1). However, the amount of UVB exposure required for synthesis of a given amount of vitamin D depends on many factors in addition to time spent outdoors. These factors include degree of skin pigmentation, body mass, degree of latitude, season, cloud cover, air pollution, area of skin exposed, and the extent of UV protection (including clothing and sunscreens).29-31 Thus, it is difficult to determine what would constitute adequate sunshine exposure for a given infant or child.
Moreover, epidemiological evidence suggests that the age at which direct sunlight exposure begins is even more important than the total sunlight exposure over a lifetime in determining the risk of skin cancer. Thus, the current AAP guidelines call for decreasing sunlight exposure in all children and for keeping infants younger than 6 months out of direct sunlight. These recommendations preclude the use of sunlight as a reliable source of vitamin D in children.32
When to recommend supplementation. The AAP recommends a daily intake of 400 IU of vitamin D for all infants, children, and adolescents. The recommended intake should be started as soon as possible after birth and can be achieved by the ingestion of 800 to 1000 mL of formula or a quart of vitamin D–fortified milk per day. A satisfactory alternative is a daily supplement that contains 400 IU of vitamin D. In infants and children who do not receive the recommended amount of formula or milk, full vitamin D supplementation is recommended.
LOOKING AHEAD
The debate about vitamin D is not over. If the values for adequate 25(OH) vitamin D3 shown in Table1 are accepted for older children and adolescents, it is very likely that a higher intake of vitamin D may be needed, especially in adolescents. However, any recommendations for increasing the dose of vitamin D intake in children will also have to consider calcium intakes, since both calcium intake and vitamin D intake are closely related to serum calcium levels.
In the spring of 2009, the IOM reconvened its expert panel to reconsider intakes of vitamin D and calcium in the US population.24 A preliminary report for public comment is expected by the spring of 2010. This report may also address the question of whether 25(OH) vitamin D3 levels should be routinely measured; however, it undoubtedly will call for more research on the issue. In any event, until the 2010 report is released, the routine measurement of 25(OH) vitamin D3 levels cannot be recommended.
Stay tuned!
References:
REFERENCES:1. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents [published correction appears in Pediatrics. 2009;123:197]. Pediatrics. 2008;122:1142-1152.
2. Ladhani S, Srinivasan L, Buchanan C, Allgrove J. Presentation of vitamin D deficiency. Arch Dis Child. 2004;89:781-784.
3. Hatun S, Ozkan B, Orbak Z, et al. Vitamin D deficiency in early infancy. J Nutr.. 2005;135:279-282.
4. Binet A, Kooh SW. Persistence of vitamin D-deficiency rickets in Toronto in the 1990s. Can J Public Health. 1996;87:227-230.
5. Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004;80(6 suppl):1697S-1705S.
6. Ward LM. Vitamin D deficiency in the 21st century: a persistent problem among Canadian infants and mothers. CMAJ. 2005;172:769-770.
7. Kreiter SR, Schwartz RP, Kirkman HN Jr, et al. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137:153-157.
8. Pettifor JM. Rickets and vitamin D deficiency in children and adolescents. Endocrinol Metab Clin North Am. 2005;34:537-553, vii.
9. Gessner BD, Plotnik J, Muth PT. 25-Hydroxyvitamin D levels among healthy children in Alaska. J Pediatr. 2003;143:434-437.
10. Testimony: US Senate Committee on Agriculture, Nutrition and Forestry hearing on "Nutrition for America's Children in Difficult Economic Times" (Sen Tom Harkin, chairman). March 4, 2009. David M. Paige, MD, MPH, The Johns Hopkins University, Baltimore. Testimony_Paige.pdf. Accessed August 6, 2009.
11. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(suppl):1080S-1086S.
12. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
13. Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261-266.
14. Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542-5548.
15. Hyppönen E, Läärä E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500-1503.
16. Greer FR, Searcy JE, Levin RS, et al. Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. J Pediatr. 1982;100:919-922.
17. Greer FR, Marshall S. Bone mineral content, serum vitamin D metabolite concentrations, and ultraviolet B light exposure in infants fed human milk with and without vitamin D2 supplements. J Pediatr. 1989;114:204-212. 18. Gordon CM, DePeter KC, Feldman HA, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531-537.
19. Institute of Medicine (IOM). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997:250-287. http://www.nap.edu/openbook.php?isbn=0309063507. Accessed August 6, 2009.
20. Cranney A, Horsley T, O'Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evidence Report/Technology Assessment No. 158. Prepared by the University of Ottawa Evidence-based Practice Center (UO-EPC) under Contract No. 290-02-0021. Rockville, MD: Agency for Healthcare Research and Quality; August 2007. AHRQ publication 07-E013.
21. Arnaud SB, Stickler GB, Haworth JC. Serum 25-hydroxyvitamin D in infantile rickets. Pediatrics. 1976;57:221-225.
22. DeLucia MC, Mitnick ME, Carpenter TO. Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for re-examining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab. 2003;88:3539-3545.
23. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(suppl): 558S-564S.
24. Shaikh U, Byrd RS, Auinger P. Vitamin and mineral supplement use by children and adolescents in 1999-2004 National Health and Nutrition Examination Survey: relationship with nutrition, food security, physical activity, and health care access. Arch Pediatr Adolesc Med. 2009;163:150-157.
25. Yetley EA, Brulé D, Cheney MC, et al. Dietary reference intakes for vitamin D: justification for a review of the 1997 values. Am J Clin Nutr. 2009;89:719-727.
26. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626-632.
27. American Academy of Pediatrics. Committee on Nutrition. The prophylactic requirement and the toxicity of vitamin D. Pediatrics. 1963;31:512-525.
28. Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med. 1992;326:1178-1181.
29. Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and its physiologic consequences. Science. 1980;210:203-205.
30. Kimlin MC, Schallhorn KA. Estimations of the human "vitamin D" UV exposure in the USA. Photochem Photobiol Sci. 2004;3:1067-1070.
31. Matsuoka LY, Wortsman J, Haddad JG, et al. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127:536-538.
32. American Academy of Pediatrics. Committee on Environmental Health. Ultraviolet light: a hazard to children. Pediatrics. 1999;104(2, pt 1):328-333.
33. Holick MF. Vitamin D status: measurement, interpretation and clinical application. Ann Epidemiol. 2009;19:73-78.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.