A Guide to Monitoring and AchievingAsthma Control in ChildrenYounger Than 5 Years
Asthma is the most prevalent chronic disease in children. In the United States, asthma affects approximately 1.4 million children younger than 5 years and causes frequent activity limitations and hospitalizations.
Asthma is the most prevalent chronic disease in children.1 In the United States, asthma affects approximately 1.4 million children younger than 5 years2 and causes frequent activity limitations3 and hospitalizations.1,4 Unfortunately, a substantial number of children in this age-group have suboptimal asthma control, demonstrated by the higher rates of emergency department (ED) visits and hospitalizations in preschool-aged children than in older children.4
In the United States, mothers of children aged 1 to 5 years with persistent weekly asthma-like symptoms (ie, cough, wheeze, breathlessness) have reported that 22% of the children had an ED visit and 11% had been hospitalized within the past 6 months.5 In 2007, approximately 851,000 children younger than 5 years had an asthma attack in the past year, which represents 61% of the children with asthma in this agegroup. 2 These findings suggest that the treatment goals of asthma are not currently being met in preschoolaged children.
The goal of asthma therapy, detailed in the 2007 National Heart, Lung, and Blood Institute and National Asthma Education and Prevention Program's Expert Panel Report 3 (EPR-3),6 is to control asthma by reducing both the impairment and risk domains. Impairment addresses the daily impact of asthma on traditional clinical indices and quality of life. Risk refers to the negative consequences of the disease or pharmacotherapy.
Impairment is reduced by preventing chronic and troublesome symptoms, minimizing short-acting β2-adrenergic agonist (SABA) use to 2 or fewer days a week, maintaining near-normal pulmonary function, maintaining normal activity levels, and meeting patients' and families' expectation of and satisfaction with asthma care. Risk is reduced by preventing recurrent exacerbations of asthma and minimizing the need for ED visits or hospitalizations, preventing reduced lung growth, and providing optimal pharmacotherapy with minimal or no adverse events. Both domains may respond differently to treatment. Treatments are selected and adjusted on the basis of the patient's level of asthma control, which is determined by assessments made by the health care provider (HCP) and caregiver.
This review provides an overview on how to assess and achieve asthma control in children younger than 5 years and presents answers based on current asthma guidelines to the following questions that arise during clinic visits:
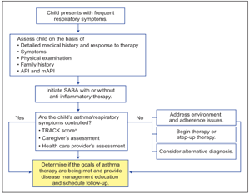
IS THE DIAGNOSIS ASTHMA?
When a child presents with frequent or chronic respiratory symptoms (Figure 1), establishing an accurate diagnosis of asthma is the first step to achieving disease control. As many as 50% to 80% of children who have asthma have symptoms before 5 years of age.6 However, there are several diagnostic challenges in this age-group of children with asthmalike symptoms. First, asthma is a heterogeneous disease with variable presentation in the frequency, type, and severity of symptoms.6,8 Second, spirometry is recommended for the diagnosis of asthma in children older than 5 years and adults, but is not generally feasible in preschool-aged children.6 Finally, asthma symptoms are common in childhood respiratory ailments, including recurrent URTIs.
Case Study: MattChief complaint. Matt is a 3-year-old boy who presents to the office with his mother because she completed TRACK online (www.asthmatracktest.com), and Matt received a TRACK score of 60. On the basis of what she read, a TRACK score lower than 80 means that Matt's breathing problems may not be under control, whereas a score of 80 or higher would suggest that his symptoms were under control. Therefore, she is worried about her child's breathing.
History of present illness. The mother states that Matt was recently seen at an after-hours clinic because he has been coughing more, especially at night, which results in his awakening. He usually is sick after an upper respiratory tract infection (URTI), which almost always “goes to his chest,” she said. After these episodes, he seems to get out of breath when he “runs around too much” at the playground. However, between these episodes, he basically is in good health.
Past medical history. A review of Matt's chart shows that he was first seen in the office for an acute respiratory infection with wheezing at 7 months of age. Over the past year, he has had 4 sick visits for respiratory complaints with diagnoses of recurrent pneumonia and reactive airway disease. He recently had an urgent care visit and was treated with nebulized albuterol and oral prednisolone for 4 days, which appeared to resolve the episode.
Allergies/medications. Matt has no known allergies. He currently uses a jet nebulizer and compressor to deliver albuterol treatments as needed. Matt's mother reports that Matt uses his albuterol medication about once or twice a week, especially during the winter months.
Family history. Matt's mother notes that she herself has had asthma since childhood and that Matt's father suffers from “sinus” allergies in the fall.
Physical examination (PE). Remarkable findings upon PE include increased nasal secretions and some mucosal swelling. The lungs are clear.

Symptoms and physical exam. In preschool-aged children, the diagnosis of asthma is based largely on clinical judgment and the assessment of symptoms and PE.9 Components of the diagnostic evaluation for this age-group include a detailed medical history; an assessment of the frequency, type, and pattern of symptoms (Table 1); as well as a PE.6
A patient's medical history allows the HCP to evaluate factors that indicate a likely diagnosis of asthma. Key indicators suggestive of asthma are wheezing, recurrent respiratory symptoms, a history of nighttime cough, symptoms that occur or worsen in the presence of a trigger, and responsiveness to a bronchodilator. Although no one indicator is diagnostic, the presence of multiple indicators increases the probability of an asthma diagnosis. Preschool-aged children with asthma often present with recurrent wheezing associated with a viral infection or complaints of recurrent pneumonia or bronchitis.10
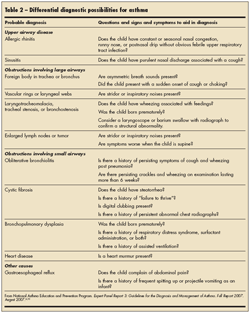
Some of the physical findings suggestive of asthma include hyperexpansion of the thorax, wheezing on chest auscultation, increased nasal secretions, mucosal swelling, atopic dermatitis, or other allergic manifestations. 6 During the evaluation, HCPs should also consider possible alternative diagnoses and perform appropriate tests, if indicated (Table 2). Some possible alternative diagnoses in infants and children include allergic rhinitis, cystic fibrosis, and gastroesophageal reflux disease. Chest radiographs, pulmonary function tests (in older children), and allergy testing are some of the additional studies that aid in the evaluation of children with recurring symptoms suggestive of asthma.
Therapeutic trials. For a child whose medical history, family history, and PE are suggestive of asthma (Figure 1),7,11 a therapeutic trial of SABA therapy (eg, albuterol), antiinflammatory therapy (eg, inhaled corticosteroid [ICS]), or both clinically helps one decide whether a child has asthma.9 Observation of symptom improvement after administration of albuterol while the child is in the office or at home during wheezing episodes also assists in establishing the diagnosis. If a SABA alone, administered by nebulizer or metered- dose inhaler (MDI) every 4 to 6 hours, does not control symptoms, the HCP should initiate a short course of an oral corticosteroid (OCS) and observe the effect of this combination on the wheezing. The role of OCSs in the future management of the child's wheezing episodes then should be decided and included in the asthma management plan.
For children with persistent asthma, as defined by the impairment or risk domains of the EPR-3, a trial of an ICS is indicated.6 Response should be monitored carefully for reduction in impairment symptoms, a reduction in the frequency and severity of exacerbations, or both. If there is no clear response to the therapeutic trial within 4 to 6 weeks, adherence to the treatment recommendation and device technique should be evaluated.6 Alternative diagnoses or adjustment of therapies should be considered if both adherence and technique are satisfactory. Marked clinical improvement during treatment with SABAs and ICSs and a return of symptoms when treatment is stopped support a diagnosis of asthma.9
CAN A CHILD OUTGROW ASTHMA?
The natural history of asthma in children younger than 5 years varies.6 Two general patterns of illness include the remission of symptoms during preschool years and the persistence of symptoms throughout childhood. The Tucson Children's Respiratory Study followed children from birth and found that of the group who had wheezing before age 3 years, 60% would report no wheezing episodes at 6 years of age.12 These children, who were called transient infant wheezers,13,14 did not have a family or personal history of atopy, had symptoms only during the first 3 years of life,12 and had diminished lung function from birth.12
The remaining 40% of the children who continued to wheeze into the school-aged years consisted of persistent nonatopic wheezers and, mostly, persistent atopic wheezers.13 Children with nonatopic wheezing did not have a family or personal history of atopy, but unlike the transient wheezers, their symptoms continued beyond 6 years of age but diminished in preadolescence.12,13 Children with the persistent atopic wheezing phenotype had atopy and a family history of asthma. These children continued to wheeze throughout the schoolaged years, often without associated viral infections, and represented the usual phenotype of childhood asthma in the 5- to 11-year age-group.
The persistence of asthma in a given child cannot be definitively predicted. 6 However, the Asthma Predictive Index (API) was developed to allow one to assess the likelihood that a child with frequent wheezing in the first 3 years of life will experience persistent asthma6,13,15 so that the child can be monitored and appropriate treatment started. Children with a positive API have a parental history of asthma or physician-diagnosed atopic dermatitis or 2 of the following: physician-diagnosed allergic rhinitis, wheezing apart from colds, or blood eosinophilia of 4% or higher.14
A retrospective analysis of 1246 patients from the Tucson Children's Respiratory Study showed that the API had a specificity of 97.4%, which is the likelihood that the schoolchildren without asthma from the original cohort would, when looking back at their histories, have had a negative API in their infancy.14,15 The study also demonstrated a positive predictive value of 76.6%, which is the probability that infants with a positive API would have had active asthma from ages 6 to 13 years, and a negative predictive value of 68.3%, which is the probability that infants with a negative API would not have experienced asthma at school age. In other words, the API was unable to correctly predict approximately 23% of persistent wheezers and 30% of transient wheezers. 14 Nonetheless, the API is currently the only available guide to assist in predicting which infants with frequent wheezing are likely to develop persistent asthma during their school years.14 Additional ongoing research is focusing on furthering phenotype- based asthma classification and individualizing treatments.16
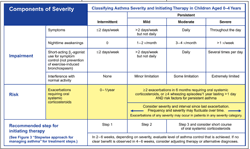
HOW SEVERE IS THE CHILD'S ASTHMA?
Once a diagnosis is made, additional information should be gathered to classify asthma severity, which is the intrinsic intensity of the disease.6 The initial classification of asthma severity is made before the child is taking long-term control medication. Severity classification is based on the domains of impairment and risk (Figure 2). Children are classified as having intermittent, mild persistent, moderate persistent, or severe persistent asthma on the basis of the assessments of both domains.
The assessment of impairment in children younger than 5 years is based on the frequency of symptoms, nighttime awakenings, and reliever use of SABAs, as well as the child's ability to engage in age-appropriate activities. The level of impairment is based on the most severe category in which any feature occurs. Recurrence of symptoms or SABA use more than 2 times a week indicates persistent asthma. Nighttime awakenings and even minor limitations in activities are also indicators of persistent disease in this age-group. Children with minimal or no impairment may have persistent disease depending on their risk domain. Therefore, it is important for HCPs who diagnose intermittent asthma in a child to periodically revaluate the child for frequency and severity of exacerbations.
The assessment of risk is based on the frequency of exacerbations.6 Generally, young children experience about 4 to 6 “colds” per year, particularly in the fall and winter months.17 In preschool- and school-aged children, hospitalizations and ED visits for asthma begin to increase every September and peak in the early fall,18-20 which is associated with an increase in viral respiratory tract infections. 18,21 For very young children, however, data to link exact frequencies of exacerbations with different levels of asthma severity are inadequate. A preschooler may have exacerbations in the absence of daily symptoms between attacks. A child who has only 1 exacerbation a year may be classified as having intermittent asthma. What is important is that children with intermittent asthma can have severe asthma exacerbations. According to the guidelines, a child with 2 or more exacerbations within 6 months requiring treatment with an OCS, even without symptoms in between, is considered to have persistent asthma. However, this recommendation is based on panel consensus judgment and clinical experience because the clinical literature addressing the subject was insufficient (Evidence level D).6
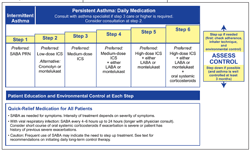
WHAT ARE THE CURRENT RECOMMENDATIONS FOR INITIAL CONTROLLER OR STEP-UP THERAPY?
Failure of over-the-counter medications to relieve a child's recurring respiratory symptoms is often the impetus for a visit to the pediatrician's office.7 Before initiating therapy, it is important for the HCP to determine what medications the child is currently taking and to establish the step of therapy the child is on, based on the 6-step treatment chart of the guidelines (Figure 3). It is also important to assess the need for these medications and whether continued treatment would be beneficial. The child already may be using an inhaled SABA as needed for quick relief of symptoms.6 The HCP should determine the frequency of inhaled SABA use to assess the need to step up therapy.
While the original API was developed to assess the likelihood that a child would experience persistent asthma, a modified API (mAPI) was used as an inclusion criterion in the Prevention of Early Asthma in Kids (PEAK) study to examine whether long-term ICS treatment would prevent disease progression in 285 children aged 2 to 3 years at high risk for persistent asthma.22
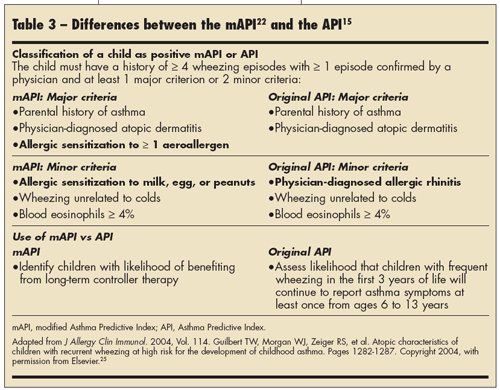
The original API was modified because allergic rhinitis is difficult to diagnose in young children and previously conducted studies have shown that early allergic sensitization to milk or eggs are predictors for developing persistent asthma. 22-24 Differences between the mAPI and the original API are described in Table 3.25 A positive mAPI requires the child to have at least 1 of 3 major risk factors or 2 of 3 minor risk factors. The PEAK study showed that children with the phenotype of a positive mAPI who received fluticasone propionate 88 µg twice daily had a significantly greater proportion of episode-free days (P = .006) and reduction in exacerbations requiring systemic corticosteroids (P < .001) compared with children who received placebo over the 2-year treatment period.26 The children were followed for 1 year after treatment was discontinued. Findings from the treatment-free year did not support a disease-altering effect after ICS discontinuation.
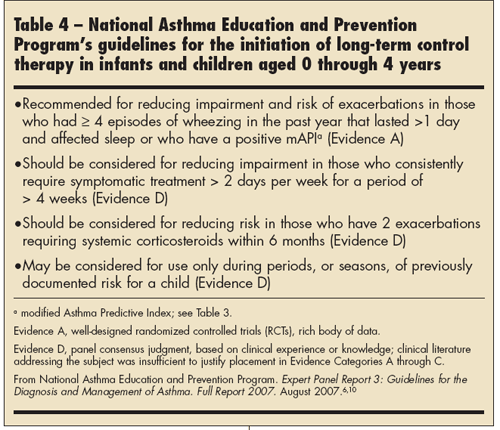
Nevertheless, the outcome of the PEAK trial led to the guideline recommendation that a long-term daily controller (ICS) be initiated for reducing risk and impairment in children younger than 5 years who have had 4 or more episodes of wheezing in the past year that lasted more than 1 day and affected sleep and who have risk factors for developing persistent asthma based on the mAPI.6,26 Many children have intermittent episodes of viral-induced wheezing, and the PEAK trial helps define which of those children with recurrent episodes will benefit from maintenance therapy. A daily long-term controller also should be considered for children with impairment (eg, persistent symptoms) or risk as outlined in Table 4.
Moreover, the guidelines recommend a stepwise treatment approach to maintain long-term asthma control (Figure 3).6 Daily controller therapy is recommended for persistent disease. An ICS is the preferred step 2 controller. A step up in therapy is warranted if asthma control is inadequate and a step down in therapy is recommended to achieve control with the minimal necessary amount of medication. A step down in therapy, with an ICS dose reduction of 25% to 50%, should be considered whenever the child's asthma has been well controlled for 3 or more months.
WHAT IS THE PREFERRED THERAPY WHEN INITIATING DAILY CONTROLLER MEDICATIONS?ICS therapy. A low-dose daily ICS is the preferred therapy when initiating daily controller treatment.6 Many clinical trials with ICSs have enrolled patients 12 years or older. The generalizability of these results to younger children has not been clearly established. However, a recent meta-analysis of 29 studies in infants and preschoolers aged 1 month to 5 years who received an ICS showed that in 2805 children from 16 studies, those who received an ICS had significantly fewer wheezing/ asthma exacerbations (18.0%) than those who received placebo (32.1%; relative risk: 0.59; 95% confidence interval: 0.52 - 0.67; I = .0001).27 Moreover, children on an ICS used significantly less albuterol and had significantly greater mean improvements from baseline in symptoms score, forced expiratory volume in 1 second, and peak expiratory flow (P = .0001).
FDA-approved ICSs for the treatment of asthma in young children are limited. Budesonide inhalation suspension administered with a jet nebulizer–compressor is approved for children aged 12 months to 8 years, has been shown to be efficacious and tolerable in this agegroup, 28 and is the only ICS indicated for use in children 3 years or younger. 6 Several other combinations of ICSs and delivery devices are available for use in infants and young children, with approval by the FDA down to 4 years of age for mometasone furoate inhalation powder and fluticasone propionate hydrofluoroalkane inhalation aerosol. Moreover, the National Asthma Education and Prevention Program does provide dosage recommendations for the use of fluticasone propionate MDI for children aged 0 to 4 years.6 Use of fluticasone MDI with a valved spacer and a face mask was studied in the PEAK trial, which showed that low-dose daily ICS therapy is not a disease modifier but does decrease exacerbations and symptom burden in preschoolaged children with risk factors based on the mAPI while the child is on treatment.26
Montelukast. An alternative approved option for this age-group is the orally administered leukotriene receptor antagonist montelukast.6 A double-blind, randomized study of 689 children aged 2 to 5 years with asthma showed that montelukast treatment over 12 weeks is significantly more effective than placebo in improving daytime asthma symptoms, the percentage of days with asthma symptoms, and the need for a rescue SABA or OCS (P ≤ .012).6,29 Montelukast also has been shown to be effective in reducing asthma exacerbations in children aged 2 to 5 years (N = 549) with viral-associated intermittent asthma.30 In school-aged children, studies show the effectiveness of ICSs to be greater than that of montelukast.6,31-33 Therefore, while both ICS and montelukast are effective, an ICS is the preferred longterm daily controller medication in preschool-aged children.6
Cromolyn. Cromolyn is available as an MDI and as a nebulizer solution, and is currently approved for use in children 2 years and older.6 However, the symptom benefits of cromolyn in preschool-aged children are inconsistent.34 According to current guidelines, cromolyn is to be used as an alternative, but not preferred, treatment at step 2 of care. If adequate asthma control is not achieved and maintained after 4 to 6 weeks, the preferred medication should be tried before stepping up therapy.6
Case Study:What Is Next for Matt?Assessment: Poorly controlled asthma. The HCP classifies Matt as having a positive API because he had 4 episodes of wheezing in the previous year with at least the most recent episode confirmed by a physician and because his mother has had asthma since childhood. On the basis of the findings from the Tucson Children's Respiratory Study, the HCP informs Matt's mother that there is an approximately 75% chance that Matt will have active asthma when he is between the ages of 6 and 13 years. Although Matt has been coughing more at night recently, his symptoms occur 2 or fewer days a week, do not usually interfere with his daily activities, and require use of albuterol 2 or fewer days a week. Despite minimal impairment, Matt's level of risk based on the number of wheezing episodes in the 12 months preceding this visit and positive mAPI classify him as a child with persistent asthma. Moreover, because of his risk level and positive mAPI, Matt meets the recommendation for a long-term controller medication, which is an increase from his current step 1 therapy (as-needed albuterol) to step 2 therapy.
Plan: Daily ICS therapy. The HCP prescribes a low-dose nebulized ICS to use once daily. This therapy is chosen because Matt appears comfortable using a nebulizer, on the basis of his home experience, while his technique with an MDI and valved holding chamber and mask appears to need further practice. An office staff member reviews the proper device technique for a nebulized ICS and ensures that Matt's mother has the proper nebulizer cup, tubing, and mask.
Matt's mother is given a written asthma management plan that lists the specific symptoms that indicate Matt's asthma is getting worse; outlines when albuterol should be given; states when it is necessary to increase therapy, including the possibility of the use of an OCS; and states when to seek urgent medical care. In 4 weeks, Matt will return for a follow-up visit and the HCP will assess whether Matt's asthma is adequately controlled.
ARE THE CHILD'S RESPIRATORY SYMPTOMS CONTROLLED?
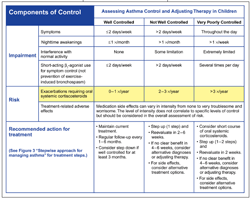
Similar to asthma severity, asthma control is defined in terms of reducing impairment and risk (Figure 4).6 In contrast to severity, asthma control reflects the degree to which asthma risks, symptoms, and limitations are minimized and goals of therapy are met. Asthma control becomes the emphasis for clinical management once therapy is initiated. Decisions to maintain or adjust therapy are based on the child's level of asthma control.
The guidelines recommend periodic assessment and monitoring of asthma control at 1- to 6-month intervals. 6 The frequency of HCP visits depends on the patient's level of asthma control and is based mainly on clinical judgment. As previously discussed, for children receiving a therapeutic trial of asthma medication, control should be assessed within 4 to 6 weeks of therapy initiation. Generally, children with intermittent or mild persistent asthma that has been controlled for 3 months or more should see their HCP every 6 months. Children with uncontrolled asthma, severe persistent asthma, or difficulty in following a treatment plan should see a physician more frequently. If step-down therapy is anticipated, the guidelines recommend a 3-month follow-up interval. The primary methods of monitoring asthma control in children are self-assessments filled out by a parent or family member and evaluation by the HCP.
The use of self-assessment tools, such as patient diaries or standardized questionnaires, is encouraged to obtain the family's perspective on the child's asthma control.6 However, currently available asthma control tools were not specifically designed for very young children. Instruments to assess asthma control have been developed for children aged 4 to 11 years,35 children and adolescents aged 5 to 1736 or 1 to 18 years,37 and adults.38-41 All of these tools were developed before the guidelines recommended the use of the risk domain for assessment of asthma control. In addition, no tool was specifically designed and validated for use by caregivers of children younger than 5 years who have respiratory symptoms consistent with asthma symptoms. For school-aged children, parents' and children's perceptions of asthma symptoms or severity can differ. 42,43 Nonetheless, a caregiver proxy for symptom assessment is necessary in younger children who are not able to complete a questionnaire.
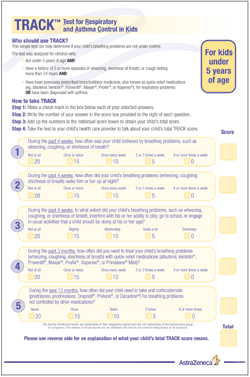
Figure 5
WHAT IS THE TEST FOR RESPIRATORY AND ASTHMA CONTROL IN KIDS (TRACKTM)?
Recently, TRACK (Figure 5) was developed and validated for caregivers of children younger than 5 years with 2 or more episodes of respiratory symptoms (eg, cough, wheeze, shortness of breath) that lasted 24 hours or more and with either physician-diagnosed asthma or bronchodilator use.7 In contrast to other validated tools, the TRACK tool encompasses both the risk and impairment domains of respiratory control consistent with current guidelines. 6,7 This tool helps to identify the children with chronic respiratory symptoms consistent with asthma symptoms who would likely be classified as having uncontrolled asthma by an HCP.
The TRACK tool was specifically designed for preschool-aged children through a qualitative and quantitative research process.7 Interviews were conducted with pediatric asthma specialists, pediatricians, and caregivers of young children with recurrent respiratory problems or asthma. On the basis of their feedback, a set of possible test questions about the frequency and severity of respiratory symptoms, the effect of these symptoms on the child's life, and health care utilization was developed. The final TRACK tool comprises 5 questions that best discriminate between the guidelines-based controlled and uncontrolled asthma ratings and that have the greatest predictive value.
The TRACK tool includes 4 impairment questions: 3 about the frequency of respiratory symptoms, activity limitations, and nighttime awakenings in the past 4 weeks, and 1 about rescue medication use in the past 3 months.7 It also includes 1 riskrelated question about OCS use in the previous year. Each question is scored from 0 to 20 points, for a total score between 0 and 100 points. Higher scores indicate better respiratory control. A TRACK score lower than 80 suggests uncontrolled asthma and may be an indication that HCPs need to provide further evaluation and possibly adjust treatment plans. A TRACK score of 80 or more suggests that the child's breathing problems are controlled.
The TRACK instrument is not a diagnostic tool but is a brief, caregiver- completed, standardized instrument that is easily administered and scored and can be used for collecting basic information about respiratory and asthma control.7 It increases caregiver and HCP awareness of potential respiratory control problems in young children. However, the role of TRACK in clinical practice and research has not yet clearly been established. An ongoing study is under way to validate the TRACK tool in test-retest situations to determine its use at follow-up clinical visits. Caregivers should base the answers of all 5 TRACK questions on their own interpretation and not seek the opinion of an HCP while answering the questions. In addition, caregivers may be able to complete TRACK either in the waiting room or examination room, although check-in time is ideal. Caregivers also may access the tool online (www.asthmatracktest.com) and complete it at home. The completion of TRACK before seeing the HCP allows for more in-depth and focused caregiver-provider discussions. The TRACK score can help to facilitate dialogue between the caregiver and the HCP to identify strategies to better manage the child's respiratory symptoms.

HCPs should not depend solely on a caregiver's assessment of the child's asthma control because families may be accustomed to their children's asthma symptoms and report good control even for very symptomatic children.44 In addition to considering the caregiver's assessment of asthma control, the clinician's assessment of asthma control should be obtained through the patient's medical history. Questions should focus on the child's signs and symptoms of asthma, level of activity, and exacerbation history.6Table 5 provides sample questions for HCPs to assess and monitor asthma control.6 In addition, the HCP should review the child's TRACK score and help the caregiver interpret the score, allowing for a specific discussion of the child's level of respiratory and asthma control based on the guidelines (Figure 4).
The child's quality of life and satisfaction with treatment should also be reviewed. HCPs should also be aware of some of the external factors that can affect asthma control. A cross-sectional study of 362 children aged 5 to 12 years with asthma who had experienced an acute exacerbation in the past year assessed demographic, family, and pediatric practice characteristics as predictors of asthma control.45 Factors associated with poor asthma control were Medicaid insurance, full-time or part-time maternal employment, and the presence of another family member in the home with asthma. The authors suggested that economic factors and a caregiver's job responsibilities may interfere with the caregiver's knowledge of the child's adherence to medication and level of asthma control. Although extrapolation to preschool- aged children cannot be made directly, the study suggests that the social history may affect asthma control.
WHAT ARE THE NEXT STEPS FOR A CHILD WITH UNCONTROLLED RESPIRATORY SYMPTOMS?
The HCP should consider coexisting conditions, incorrect diagnosis, new or increased exposures to allergens or irritants, and psychosocial problems that could contribute to suboptimal asthma or respiratory control.6 In children with asthma, inhalant allergens can increase airway inflammation and symptoms. Consequently, reducing a child's exposure to such allergens can significantly reduce asthma symptoms and the need for medications.
The HCP should question caregivers on the child's exposure to allergens such as those associated with pets, mold, moisture or dampness, dust mites, tobacco smoke, unvented stoves or heaters, and medication sensitivities. By using the medical history and skin testing or in vitro testing, with the help of a pediatric allergist, the child's specific allergen sensitivities can be determined. Methods to control asthma by reducing the child's exposure to environmental allergens include removing the pet from the house or, at minimum, keeping the pet out of the child's bedroom, minimizing the child's exposure to tobacco smoke, and closing the windows during periods of peak pollen levels. Aerobiology varies with the region, and molds such as Alternaria are major seasonal causes of asthma in many parts of the United States.
In addition, the evaluation and treatment of comorbidities such as obesity and gastroesophageal reflux disease are important because these comorbidities represent independent risk factors that can increase the severity of childhood asthma and cause poor response to treatment. Assessment of what asthma medications the child is taking and adherence to those medications is also a key component to assessing control. As previously discussed, the HCP should determine whether the child is taking any medications for his/her asthma. If the child is, inquire about dosing; inhaler or nebulizer techniques, if appropriate; and adherence by discussing the child's normal daily routine (Table 6). Consultation with an asthma specialist is recommended for children who need step 3 care or higher and should be considered for those who need step 2 care (Figure 3).

HOW CAN CAREGIVERS HELP CHILDREN ACHIEVE ASTHMA CONTROL?
To facilitate asthma control, a detailed written asthma management plan should be provided to caregivers of children with either intermittent or persistent asthma. A plan is especially important for children with intermittent disease and a history of exacerbations because these patients can experience sudden and life-threatening exacerbations.6 The action plan should include specific symptoms indicative of worsening asthma, recommendations for SABA and OCS use, and when to seek medical care.
Once asthma is diagnosed in a child, asthma self-management education should be initiated and continued throughout the child's asthma care. Asthma self-management education provides patients and caregivers with the skills necessary to control asthma and improve symptoms. 6 The goals of therapy should be established between the caregiver and the HCP. Caregivers should be educated on how medications work and on proper device technique. Addressing caregivers' concerns and encouraging treatment adherence are important because caregivers play a major role in establishing and ensuring that the child's asthma is controlled.
After each HCP visit, it is also important for the caregiver to schedule a follow-up visit to continually ensure that the child's asthma is well controlled (Figure 1). The caregiver should ensure that a written asthma action plan is in place at all times and should review it with the HCP at each follow-up visit to determine whether any adjustment to the plan is necessary.6 Caregivers should also note any changes in the severity or frequency of the child's asthma symptoms, especially if wheezing occurs in the absence of a cold or with exercise, and should seek the HCP's advice if such changes are observed.
Case Study: Follow-upAfter 4 weeks, Matt returns to the HCP's office for a follow-up visit. According to Matt's mother, his symptoms seem to be controlled. He has had symptoms of coughing and wheezing only once since his last visit and has not required any oral corticosteroids. Matt is sleeping through the night and has been taking his medications as prescribed. His mother reports that he has needed to use his albuterol only once in the past 2 weeks. Matt's PE results are normal. The HCP determines that Matt's asthma is well controlled (Figure 4) and continues to prescribe a nebulized ICS. The HCP reviews the written action plan with Matt and his mother, reviews device technique, and schedules a follow-up appointment in 3 months to review asthma control and medication requirements. Referral is made to the pediatric allergist for definitive allergy testing and collaborative consultation on longterm management.
CONCLUSIONS
The diagnosis and treatment of asthma in preschool-aged children is challenging. The role of asthma phenotypes in making treatment decisions for preschool-aged children is a focus of current research. Periodic and ongoing monitoring is necessary to ensure asthma control, defined by the degree to which both the impairment and risk domains of asthma are minimized by treatment. In these children, assessments by clinicians and caregivers are the primary means of monitoring asthma control. Allergy testing is indicated in young children with asthma. TRACK is a new validated and easy-to-administer caregiver completed questionnaire that can be used to aid caregivers and clinicians in evaluating respiratory control in children younger than 5 years with respiratory symptoms consistent with asthma symptoms. The role of TRACK in managing long-term, ongoing asthma is currently being studied. Caregivers have a very active role in managing a child's asthma control. The HCP should provide the caregiver with a written asthma management plan and asthma self-management education. Follow-up at regular intervals is necessary to maintain control of asthma in preschool-aged children.
References:
REFERENCES:
1. American Lung Association. Childhood AsthmaOverview. http://www.lungusa.org/site/pp.asp?c=dvLUK9O0E&b=22782. Published April 2008.Accessed January 16, 2009.
2. American Lung Association Epidemiology andStatistics Unit Research and Program ServicesDivision. Trends in Asthma Morbidity and Mortality.http://www.lungusa.org/atf/cf/%7B7A8D42C2-FCCA-4604-8ADE-7F5D5E762256%7D/ASTHMA%20JAN%202009.PDF. Published January 2009.Accessed February 13, 2009.
3. GlaxoSmithKline. Children & Asthma in America.Executive Summary. http://www.asthmainamerica.com/children_index.html. Accessed March 18, 2009.
4. Akinbami L; Centers for Disease Control andPrevention National Center for Health Statistics. Thestate of childhood asthma, United States, 1980-2005.Adv Data. 2006;381:1-24.
5. Bisgaard H, Szefler S. Prevalence of asthma-likesymptoms in young children. Pediatr Pulmonol.2007;42:723-728.
6. National Asthma Education and PreventionProgram. Expert Panel Report 3: Guidelines for theDiagnosis and Management of Asthma. Full Report2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed July 22, 2009.
7. Murphy KR, Zeiger RS, Kosinski M, et al. Test forrespiratory and asthma control in kids (TRACK): acaregiver-completed questionnaire for preschool-agedchildren. J Allergy Clin Immunol. 2009;123:833-9.e9.
8. Chipps BE, Spahn JD, Sorkness CA, et al. Variabilityin asthma severity in pediatric subjects with asthmapreviously receiving short-acting beta2-agonists.J Pediatr. 2006;148:517-521.
9. Global Initiative for Asthma (GINA). GlobalStrategy for the Diagnosis and Management ofAsthma in Children 5 Years and Younger. http://www.ginasthma.com. Accessed July 2009.
10. Eigen H. Differential diagnosis and treatmentof wheezing and asthma in young children. ClinPediatr (Phila). 2008;47:735-743.
11. Pedersen S. Preschool asthma-not so easy todiagnose [letter]. Prim Care Respir J. 2007;16:4-6.
12. Martinez FD, Wright AL, Taussig LM, et al.Asthma and wheezing in the first six years of life.The Group Health Medical Associates. N Engl JMed. 1995;332:133-138.
13. Taussig LM, Wright AL, Holberg CJ, et al.Tucson Children's Respiratory Study: 1980 to present.J Allergy Clin Immunol. 2003;111:661-675.
14. Castro-Rodriguez JA, Garcia-Marcos L. Wheezingand asthma in childhood: an epidemiology approach.Allergol Immunopathol (Madr). 2008;36:280-290.
15. Castro-RodrÃguez JA, Holberg CJ, Wright AL,Martinez FD. A clinical index to define risk of asthmain young children with recurrent wheezing. AmJ Respir Crit Care Med. 2000;162(4 pt 1):1403-1406.
16. Bush A. Update in pediatric lung disease 2008.Am J Respir Crit Care Med. 2009;179:637-649.
17. Heikkinen T, Järvinen A. The common cold.Lancet. 2003;361:51-59.
18. Johnston NW, Johnston SL, Norman GR, et al.The September epidemic of asthma hospitalization:school children as disease vectors [published correctionappears in J Allergy Clin Immunol. 2007;120:47]. J Allergy Clin Immunol. 2006;117:557-562.
19. Van Dole KB, Swern AS, Newcomb K, NelsenL. Seasonal patterns in health care use and pharmaceuticalclaims for asthma prescriptions for preschool-and school-aged children. Ann AllergyAsthma Immunol. 2009;102;198-204.
20. Sears MR, Johnston NW. Understanding theSeptember asthma epidemic. J Allergy Clin Immunol.2007;120:526-529.
21. Dales RE, Schweitzer I, Toogood JH, et al.Respiratory infections and the autumn increase inasthma morbidity. Eur Respir J. 1996;9:72-77.
22. Guilbert TW, Morgan WJ, Krawiec M, et al.The Prevention of Early Asthma in Kids study:design, rationale and methods for the ChildhoodAsthma Research and Education network. ControlClin Trials. 2004;25:286-310.
23. Nickel R, Kulig M, Forster J, et al. Sensitizationto hen's egg at the age of twelve months is predictivefor allergic sensitization to common indoor andoutdoor allergens at the age of three years. J AllergyClin Immunol. 1997;99:613-617.
24. Kulig M, Bergmann R, Tacke U, et al; The MASStudy Group, Germany. Long-lasting sensitization tofood during the first two years precedes allergic airwaydisease. Pediatr Allergy Immunol. 1998;9:61-67.
25. Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopiccharacteristics of children with recurrent wheezingat high risk for the development of childhood asthma.J Allergy Clin Immunol. 2004;114:1282-1287.
26. Guilbert TW, Morgan WJ, Zeiger RS, et al.Long-term inhaled corticosteroids in preschool childrenat high risk for asthma. N Engl J Med. 2006;354:1985-1997.
27. Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaledcorticosteroids in infants and preschoolers withrecurrent wheezing and asthma: a systematic reviewwith meta-analysis. Pediatrics. 2009;123:e519-e525.
28. Szefler SJ, Eigen H. Budesonide inhalation suspension:a nebulized corticosteroid for persistentasthma. J Allergy Clin Immunol. 2002;109:730-742.
29. Knorr B, Franchi LM, Bisgaard H, et al. Montelukast,a leukotriene receptor antagonist, for thetreatment of persistent asthma in children aged 2 to5 years. Pediatrics. 2001;108:e48.
30. Bisgaard H, Zielen S, Garcia-Garcia ML, et al.Montelukast reduces asthma exacerbations in2- to 5-year-old children with intermittent asthma.Am J Respir Crit Care Med. 2005;171:315â322.
31. Garcia Garcia ML, Wahn U, Gilles L, et al.Montelukast, compared with fluticasone, for controlof asthma among 6- to 14-year-old patients with mildasthma: the MOSAIC study [published correctionappears in Pediatrics. 2005;116:1058]. Pediatrics.2005;116:360-369.
32. Ostrom NK, Decotiis BA, Lincourt WR, et al.Comparative efficacy and safety of low-dose fluticasonepropionate and montelukast in children withpersistent asthma. J Pediatr. 2005;147:213-220.
33. Sorkness CA, Lemanske RF Jr, Mauger DT, etal; Childhood Asthma Research and Education Networkof the National Heart, Lung, and Blood Institute.Long-term comparison of 3 controller regimensfor mild-moderate persistent childhood asthma: thePediatric Asthma Controller Trial [published correctionappears in J Allergy Clin Immunol. 2007;120:285].J Allergy Clin Immunol. 2007;119:64-72.
34. Tasche MJ, Uijen JH, Bernsen RM, et al.Inhaled disodium cromoglycate (DSCG) as maintenancetherapy in children with asthma: a systematicreview. Thorax. 2000;55:913-920.
35. Liu AH, Zeiger R, Sorkness C, et al. Developmentand cross-sectional validation of the ChildhoodAsthma Control Test. J Allergy Clin Immunol. 2007;119:817-825.
36. Skinner EA, Diette GB, Algatt-Bergstrom PJ,et al. The Asthma Therapy Assessment Questionnaire(ATAQ) for children and adolescents. Dis Manag.2004;7:305-313.
37. Zorc JJ, Pawlowski NA, Allen JL, et al. Developmentand validation of an instrument to measureasthma symptom control in children. J Asthma.2006;43:753-758.
38. Juniper EF, O'Byrne PM, Guyatt GH, et al. Developmentand validation of a questionnaire to measureasthma control. Eur Respir J. 1999;14:902-907.
39. Juniper EF, O'Byrne PM, Ferrie PJ, et al.Measuring asthma control. Clinic questionnaire ordaily diary? Am J Respir Crit Care Med. 2000;162(4 pt 1):1330-1334.
40. Nathan RA, Sorkness CA, Kosinski M, et al.Development of the asthma control test: a survey forassessing asthma control. J Allergy Clin Immunol.2004;113:59-65.
41. Vollmer WM, Markson LE, O'Connor E, et al.Association of asthma control with health careutilization and quality of life. Am J Respir Crit CareMed. 1999;160(5 pt 1):1647-1652.
42. Lara M, Duan N, Sherbourne C, et al. Differencesbetween child and parent reports of symptoms amongLatino children with asthma. Pediatrics. 1998;102:E68.
43. Erickson SR, Munzenberger PJ, Plante MJ,et al. Influence of sociodemographics on the healthrelatedquality of life of pediatric patients with asthmaand their caregivers. J Asthma. 2002;39:107-117.
44. Halterman JS, McConnochie KM, Conn KM,et al. A potential pitfall in provider assessments ofthe quality of asthma control. Ambul Pediatr. 2003;3:102-105.
45. Bloomberg GR, Banister C, Sterkel R, et al.Socioeconomic, family, and pediatric practice factorsthat affect level of asthma control. Pediatrics. 2009;123:829-835.