The future of vaccinology
A look at where vaccine technologies will be going.
No aspect of pediatric medicine has been as impactful on the health and well-being of our patients and communities as the vaccines that now routinely prevent 16 diseases that were once commonplace.1 Current vaccines include those based on live attenuated viruses (eg, varicella, rotovirus), killed whole organism (eg, rabies, hepatitis A), polysaccharide or native proteins (eg, pneumococcal, meningococcal), and recombinant or whole molecular modification (eg, hepatitis B, herpes zoster).
Today nearly 260 vaccines are in development (Figure 1). Many of these are directed at infectious diseases, but many novel vaccines are being developed to treat or prevent allergies, autoimmune disorders, cancers, and even Alzheimer’s disease.1
Figure 1
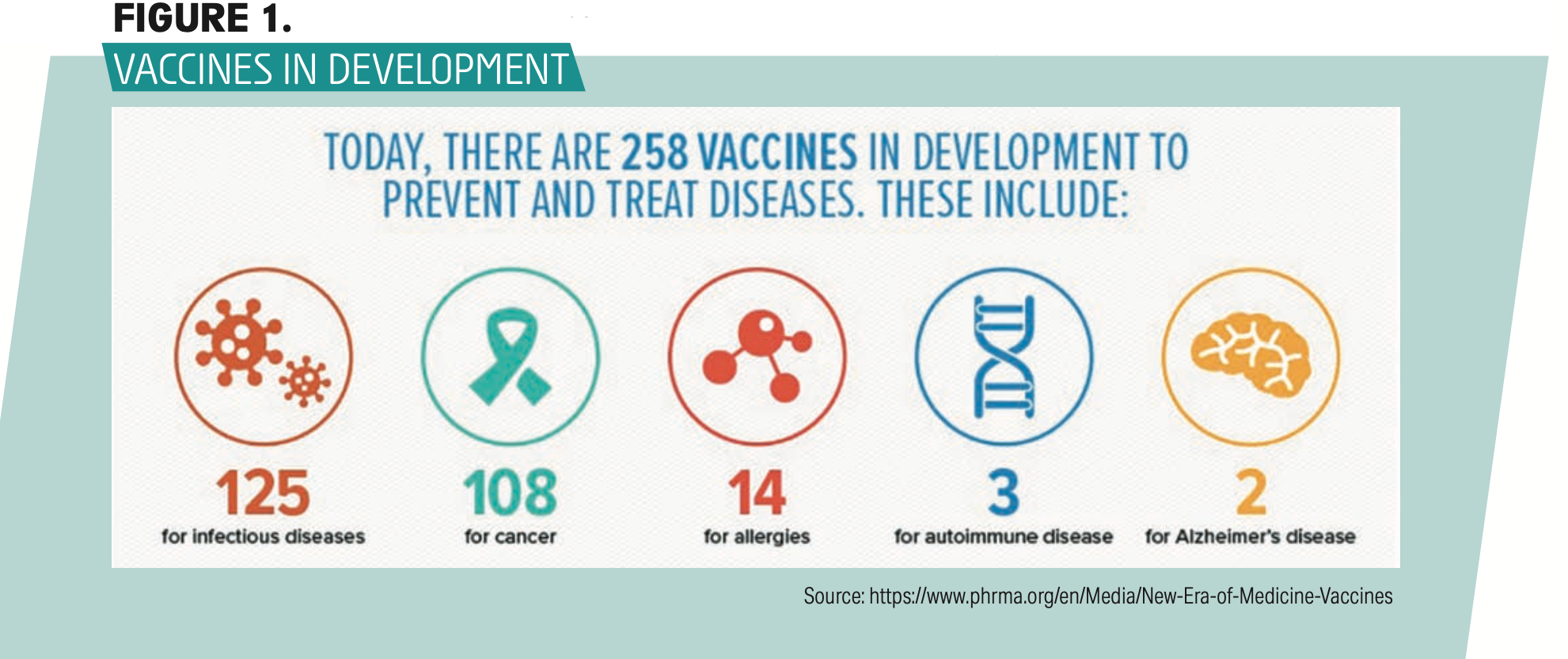
Following the emergence of severe acute respiratory syndrome (SARS) in 2003 and Middle East respiratory syndrome (MERS) in 2012, both deadly coronavirus infections, investigators spent subsequent years developing and perfecting innovative vaccines including adenovirus vector vaccines and messenger RNA (mRNA) vaccines. Both vaccine types were rapidly adapted to combat the SARS-CoV-2 pandemic once the Chinese published the genetic sequence of SARS-CoV-2 in January 2020.2 These vaccines instruct cells to manufacture specific antigens—ie, the spike proteins in the case of the SARS-CoV-2 virus—so that the immune system manufactures spike protein antibodies. As of this writing, the United States has deployed 2 mRNA vaccines (Pfizer and Moderna), as well as a genetically engineered adenovirus vector vaccine (Johnson & Johnson). Both vaccine technologies can easily be modified to target a litany of other viruses.
To date, several adenovirus vector vaccines for HIV, Ebola virus, influenza virus, Mycobacterium tuberculosis, and Plasmodium falciparum are under human clinical trials, and others are being developed against rabies, dengue virus, and MERS. Currently, mRNA-based vaccines are in development against HIV, rabies, Zika, cytomegalovirus, respiratory syncytial virus (RSV), Ebola, and others.
These vaccine technologies have also shown great promise against several types of cancer. More than 150 clinical trials assessing mRNA vaccines and more than 200 clinical trials involving adenovirus vector vaccines for infectious diseases and cancers are currently ongoing, according to ClinicalTrials.gov. It is even anticipated that a universal flu vaccine based on these technologies will be developed, and it will no longer require seasonal modifications because it will target stable antigens among influenza viruses.
It is also predicted that there will be new vaccines based on virus like particles (VLPs). These are artificial viruses made up of copies of 1 or more viral proteins that self-assemble into nanoparticles. Current VLP vaccines include the hepatitis B vaccine (Energix-B) and the human papillomavirus vaccine (Gardasil). VLP vaccines are also being developed for chikungunya, Japanese encephalitis, yellow fever, Zika, and malaria, among other diseases.3
Yet another new type of vaccine— against allergic asthma—is being investigated. Allergic asthma is characterized by mucus secretion, airway eosinophilia, and elevated levels of IgE antibodies and increased levels of cytokines IL-3 and IL-4. Recently, researchers produced an experimental conjugate vaccine against these interleukins that in animal models have shown prolonged reduction of IgE levels, airway hyperresponsiveness, eosinophilia, and mucus production.
New ways to administer vaccines
Additionally, there are new vaccine delivery systems under development. The most common method of immunizing children to date has been the direct injection of vaccines either intramuscularly or subcutaneously. Several years ago, a live attenuated influenza intranasal vaccine was developed, which has benefited countless needle-phobic children and adults. Now Altimmune has developed an adenovirus-vectored intranasal vaccine, now in phase I clinical trials, that generates a broad IgG, mucosal IgA, and T- cell response to SARS-CoV-2 and is stable at room temperature.5 Altimmune is also in clinical trials for intranasal vaccines for anthrax and influenza.
Several companies are developing a microneedle patch to administer vaccines. The microneedles are impregnated with vaccine that targets immune cells in the dermis.6 Best of all, these are pain free and stable at room temperature. The patch is applied via a preloaded applicator and removed after a few minutes, leaving the vaccine-impregnated tips embedded in the dermis, releasing vaccine over time. These patches are being investigated as a potential alternative to intramuscular vaccines for influenza and COVID-19.
Two more vaccine delivery systems may change the way patients are immunized. An oral vaccine mechanism, Vector-Adjuvant-Antigen Standardized Technology (VAAST), which tar- gets mucosal IgA is being perfected by Vaxart. VAAST has shown promising results for H5N1 influenza, H1N1 influenza, influenza B, norovirus, and RSV. The second is a vaccine capsule filled with nanoparticles, injected subcutaneously, that would slowly release the vaccine over time, potentially eliminating the need for immunization booster doses.7 Enesi Pharma is developing a universal vaccine implant (UVI) using their ImplaVax needle-free delivery device to inject each UVI up to 7 mm into the dermis (Figure 2). Preclinical studies have shown this method to be as effective as intramuscular on subcutaneous injections, with less pain associated with injection, according to the company.
Figure 2
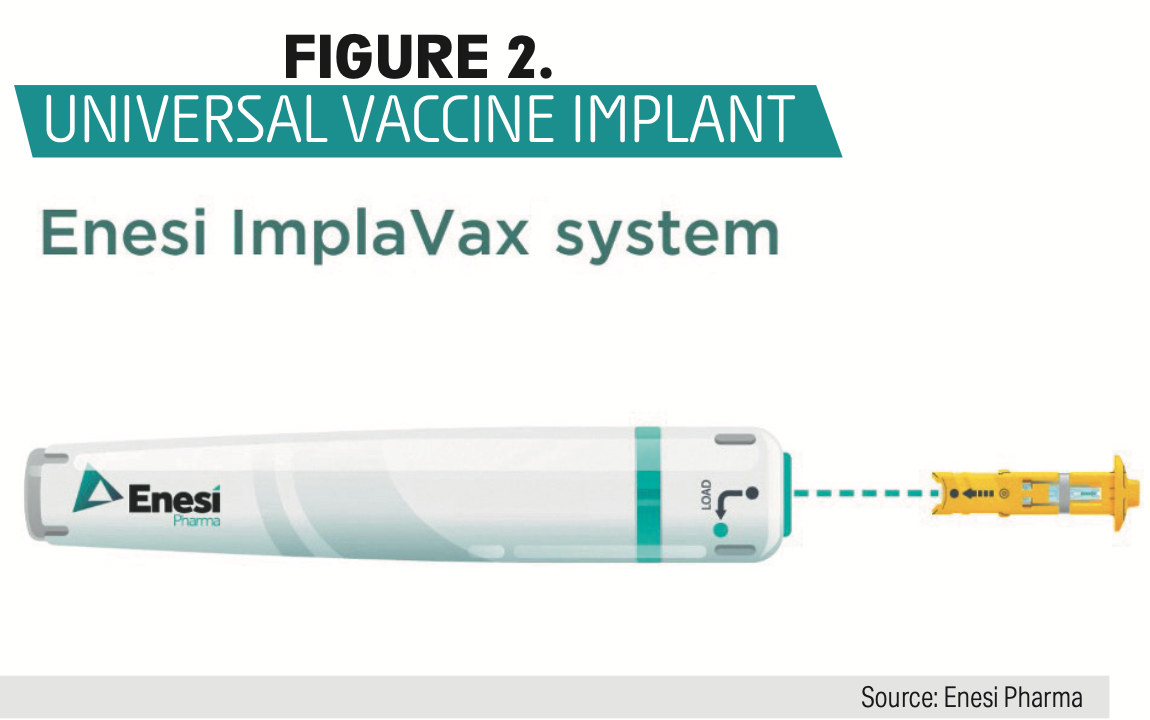
Conclusion
Vaccine technologies in development may facilitate a rapid and effective response to new threats and likely will target not only infectious diseases but also allergies, autoimmune conditions, and cancers. Additionally, new vaccine delivery systems will overcome production and storage obstacles that have slowed the response to the COVID-19 pandemic. Pediatricians and parents, recognizing how much vaccines improve the health and well-being of young patients, have much to look forward to.
References
1. New era of medicine: vaccines. Pharmaceutical Research and Manufacturers of America.Accessed August 23, 2021. https://www.phrma.org/en/Media/New-Era-of-Medicine-Vaccines
2. Sah R, Rodriguez-Morales AJ, Jha R, et al. 2020. Complete genome sequence of a 2019 novel coronavirus (SARS-CoV-2) strain isolated in Nepal. Microbiol Resour Announc. 2020;9(11):e00169-20. doi:10.1128/MRA.00169-20
3. Garg H, Mehmetoglu-Gurbuz T, Joshi A. Virus Like Particles (VLP) as multivalent vaccine candidate against chikungunya, Japanese encephalitis, yellow fever and zika virus. Sci Rep. 2020;10(1):4017. doi:10.1038/s41598-020-61103-1
4. Conde E, Bertrand R, Balbino B, et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat Commun. 2021;12(1):2574. doi:10.1038/s41467-021-22834-5
5. King RG, Silva-Sanchez A, Peel JN, et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv. Priprint posted online October 11, 2020. doi:10.1101/2020.10.10.331348
6. Li Y, Hu X, Dong Z, et al. Dissolving microneedle arrays with optimized needle geometry for transcutaneous immunization. Eur J Pharm Sci. 2020;(151):105361. doi:10.1016/j.ejps.2020.105361
7. Bobbala S, Hook S. Vaccine implants: current status and recent advancements. Emerg Top Life Sci. 2020;4(3):319-330. doi:10.1042/ETLS20200164
