Urethral catheterization: The case for caution
Catheterizing infants to monitor urine production has become so common that practitioners can lose sight of the procedure's dangers. These guidelines will help prevent urethral injuries.
Urethral catheterization: The case for caution
By Timothy P. Bukowski, MD, and Andrew L. Freedman, MD
Catheterizing infants to monitor urine production has becomeso common that practitioners can lose sight of the procedure's dangers.These guidelines will help prevent urethral injuries.
During the last decade, we have made tremendous strides in the medicaland surgical treatment of the neonate and infant, and the growing use ofinvasive monitoring equipment is an integral part of this progress. Theplacement of a urinary drainage catheter for continuous monitoring of urineproduction or to obtain urine for analysis or culture has become as routineas using cardiac leads or a temperature probe. A casual attitude towardthe use of catheters, however, can lead to overutilization, a disregardfor risks, and improper placement of the catheter by inadequately trainedpersonnel.
Unlike an external monitor, the urinary drainage catheter is invasiveand has the potential for urethral injury. Common complications includeurinary tract infection, urethritis, bladder spasms, hematuria, meatal stenosis,urethral tear or false passage, and most significant, urethral stricture,which can lead to a lifetime of voiding difficulties requiring multiplecomplex reconstructive surgeries.13 In one series of repairedurethral strictures, 5% (three of 57) were caused by placement of a urethralcatheter.4 Although these problems can occur at any age, boys2 years old or younger are most vulnerable. Bladder perforation, ureteralobstruction, and anaphylaxis caused by the prep agent or latex in the catheter,while uncommon, are also possible.5,6
The best strategy fordealing with these problems is, of course, to preventthem. This can be accomplished by appropriate catheter selection, gentleplacement by personnel with adequate training and supervision, proper consultationif difficulty with placement or removal is encountered, secure immobilizationof the catheter, and prompt removal when it is no longer necessary.
Proving the point
Three recent cases of catheter injury in infants illustrate preventableproblems:
Case 1. A newborn male with oliguria and mild respiratory distress wasadmitted to the newborn intensive care unit for observation. A #8 Frenchballoon catheter was inserted in the urethra to monitor fluid. After theballoon was inflated, a bloody urethral discharge was seen at the meatus.The catheter was removed and replaced with a #5 French feeding tube. Bleedingpersisted, and a balloon catheter was reinserted, pulled to the bladderneck with light tension, and gently wrapped with gauze until the bleedingstopped (about 10 to 15 minutes). Four days later, a voiding cystourethrogram(VCUG) revealed extravasation of contrast into the bulbar urethra (Figure1). The child also had grade 2 reflux, which was thought to be transitoryand caused by Foley balloon irritation. The Foley catheter was removed andthe patient voided without difficulty.
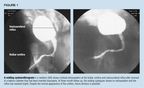
This case illustrates the danger of inflating the balloon before thecatheter is inserted completely into the bladder. As the eye-hole is distalto the balloon, urine can be expressed even if just the tip of the catheteris in the bladder and the balloon remains in the prostatic urethra. A ballooncatheter should be inserted to the stem of the balloon inflation port beforeit is inflated. Occasionally, when the urethra or prostate has bled, gentletraction on the catheter provides a tamponade effect. (One can tape thecatheter to the leg.) This is a useful maneuver in selected cases but cancause glans ischemia with necrosis if the catheter is under too much tensionor is left in place too long.
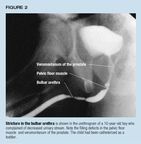
Urine extravasation put this patient at risk for stricture, necessitatinglong-term follow- up. Figure 2 shows a stricture in a 10-year-old boy witha history of catheterization.
Case 2. A 2-week-old boy with severe abdominal distention was admittedthrough the emergency department, where a balloon catheter was placed. Initiallythe serum creatinine did not fall from 2.3 mg/dL, and imaging studies revealeda rupture in the collecting system of the left kidney with a large retroperitonealurinoma. VCUG showed posterior urethral valves but no vesicoureteral reflux.The balloon was at the bladder neck and was overinflated; this conditionwas thought to contribute to ureteral obstruction and continued urine extravasation(Figure 3). The posterior urethral valves were destroyed with electric sparksgenerated by a high frequency current (fulguration), and a #8 French feedingtube was left in place for two days. The serum creatinine fell immediately,and continued to decline--to a low of 0.5 mg/dL--after the tube was removed.The hole in the kidney spontaneously sealed, and the retroperitoneal urinomaeventually reabsorbed.
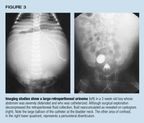
This case demonstrates that a catheter balloon can obstruct the uretereither mechanically or because of bladder spasm. The trigone is small comparedwith the balloon, especially in the neonate. The catheter balloon may alsohave temporarily prevented reflux.
Case 3. A 1-month-old boy with a prenatal diagnosis of right hydronephrosiswas catheterized for an outpatient VCUG. When the #10 French single-lumencatheter was placed, medical personnel noticed that the catheter tip wasprotruding from the urethral opening, indicating that the catheter had foldedon itself. The catheter could not be withdrawn, and an ill-advised firmtug only wedged it more tightly. A plain film showed that the catheter wastightly folded in two in the urethra (Figure 4). Gentle manipulation underanesthesia was unsuccessful; opening the bladder and trying to draw thefolded portion of the catheter into it did not work either. Finally, aftera perineal urethrostomy exposed the tightly folded and impacted catheter,the tube was unfolded with gentle teasing and removed. A fresh siliconecatheter was placed in the urethra and the urethrostomy carefully closed.Fortunately the child has not developed a stricture.
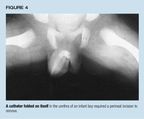
A catheter tube is most likely to fold on itself when the child's externalsphincter is tense, for example during a crying spell. When a feeding tubeis used as a catheter, its long length predisposes it to coiling and possiblyknotting in the bladder or urethra. One should advance the catheter onlyuntil urine returns and tape the catheter in position. If the catheter meetsresistance at the external sphincter, exerting gentle pressure as the childtakes a breath or "blows out a candle" may allow it to pass. Ifa coiled catheter knots, inserting a thin wire may help to unfurl the knot.
How to avoid complications
To minimize the risks of urethral catheterization, practitioners shouldbe familiar with the following guidelines for catheter selection and placement.
Make sure a catheter is truly needed. A bladder ultrasound is noninvasiveand provides an accurate estimate of volume. It can be a good alternativeto catheterization when urinary obstruction or hydration status is a concern.When infection is a possibility, a bag-collected specimen is useful forurinalysis, which can suggest infection if a spun microscopic specimen revealspyuria and bacteria. A catheterized specimen can then be obtained for culturingbefore antibiotic therapy is instituted.
Choose the right catheter. Each of the many available catheters has itsadmirers. For the young child, the two most popular are a self-retainingballoon catheter (Foley type) and an infant feeding tube. While latex cathetersare readily available for adults, the flexibility of small latex cathetersand their high wall-thickness-to-diameter ratio make them a poor choicefor infants and children. In addition, studies in adults have shown a higherrate of urethritis and stricture with latex catheters than with Silasticor silicone catheters.7 Silastic or plastic catheters are essentialin children with a history of multiple surgeries for conditions such asmyelomeningocele, exstrophy, prune belly syndrome, and cloacal malformationbecause these patients are at risk of latex sensitivity.6
In infants, we prefer an infant feeding tube to the balloon catheter,which has many disadvantages:
- Because of its two internal lumens, the balloon catheter must have a larger external diameter than might otherwise be necessary for adequate drainage. The smallest Foley typically available is #6 French, while feeding tubes as small as #3.5 French are easy to obtain.
- The stiff stilet provided for insertion of the small balloon catheters may increase the risk of a false passage, or even bladder perforation.8
- The balloon itself frequently causes serious urethral injury, usually when it is mistakenly inflated while still in the urethra.
- The balloon greatly increases the likelihood and severity of bladder spasms. Anticholinergics (for example, oxybutinin, 0.2 mg/kg taken orally twice a day) may bring relief but have side effects of their own.
Unlike adults, infants usually do not need self-retaining catheters.Hospitalized babies are too young to walk or even move about much in bed,and they are more likely than adults to be seriously ill, making it mucheasier to secure the catheter without a retention balloon. With proper taping,the catheter is unlikely to become dislodged.
We see no compelling reason for their routine use, but when balloon cathetersare necessary we recommend that they be made of silicone or coated withTeflon to reduce irritation and inflammation of the urethral mucosa.7,9
The catheter should be of the smallest diameter that provides adequatedrainage. For simply draining clear urine in a neonate, a #3.5 or #5 Frenchfeeding tube should suffice. In the older infant or young child, a #5 or#8 French feeding tube is appropriate. If the urine is bloody or it is necessaryto irrigate mucous, such as after a bladder augmentation, a larger cathetermay be necessary. Infants older than 6 months and children up to age 3 generallytolerate a #8 or #10 French catheter easily. Most children from 4 to 10years old should accept a #10 or #12 French tube, while children older than11 can generally accommodate up to #14 French.10,11
Catheter choice may also be influenced by suspected urologic pathology.Patients with posterior urethral valves or congenital urethral stenosismay have a thickened, high bladder neck, so a coudé tip cathetermay be helpful. The elbowed tip also makes it easier to traverse curvesin the male urethra (Figure 5).
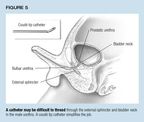
Take care in placing the catheter. It's best to have a sterile fieldwhen placing a catheter, though the preparations may heighten anxiety inolder children, causing the external sphincter to tighten and making catheterplacement more difficult. In an uncircumcised infant, withdraw the foreskinonly as far as it moves easily and don't tear adhesions. If the prepuceis retractile, put it back in the natural position after inserting the catheter,to avoid possible painful swelling of the glans (paraphimosis).
Lubricate liberally, and pass the catheter with slow gentle pressure.Any resistance or blood is a signal to stop and call for urologic consultation.Causes of resistance include previous stricture, creation of a false passage,voluntary or involuntary external urethral sphincter spasm, vagina masculinusin boys with hypospadias, or a common urogenital sinus in patients withambiguous genitalia.
Patients who have undergone urethral reconstruction, as for hypospadiasor epispadias, need special care, as do those few children without a patenturethral canal because of major reconstruction or diversion.
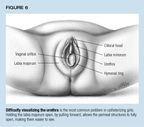
To help locate the urethra in girls, a useful maneuver is to have anassistant grasp each labia majorum and gently pull forward, forming a cave.The hymen and urethra will drop open, as shown in Figure 6.12
As explained in Case 2 above, occasionally a feeding tube catheter willknot, most often in boys under 2 years of age1315 when toolong a piece of tubing is used. Because the tube is narrow, urine movesthrough it slowly and may not appear immediately, deceiving the practitionerinto inserting the catheter farther than necessary. The catheter then circlesaround the bladder, occasionally knotting, or finds its way out the bladderneck and kinks in the urethra. The best way to prevent this problem is toplace only the necessary length of tubing in the bladder and then simplywait for urine to appear or gently depress the suprapubic region. A ruleof thumb for the proper catheter length is twice the length of the penisplus four centimeters. On those uncommon occasions when there is no urinereturn, gentle irrigation clears the tubing of lubricant.
The balloon of a balloon catheter should not be inflated until the catheteris unequivocally in the bladder--that is, when a pediatric catheter of theappropriate size has been advanced into the bladder to the valve stem. Placementcan be confirmed by irrigation if no urine drainage is apparent. Inflatethe balloon 1 mL for each year of the child's life up to 3 mL; use sterilewater, not saline or intravenous fluid, to prevent formation of small crystalsthat could block the balloon's lumen.
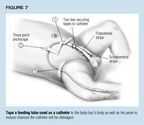
Immobilize the catheter. Tape the catheter securely when insertion iscomplete. This not only prevents the catheter from being dislodged but mayreduce friction and discomfort. For a feeding tube catherizing a boy, applythe tape longitudinally along the penile shaft as "racing stripes,"or use a clear plastic adherent dressing, such as Tegaderm, Opsite, or Biocclusive.Secure the extra length of catheter in a three-quarter circle arch acrossthe abdomen, using lengths of adhesive tape at three points. At least twolengths should be perpendicular to each other, as shown in Figure 7. Ina girl, the catheter can be taped to the inside thigh. A balloon cathetershould be taped to the leg at the catheter's hub and should be long enoughfor the child to flex at the hips without pulling the catheter. Taping thetubing portion allows the catheter to twist, and it may break where thetube meets the hub. This is especially likely if an adult drainage bag withthick tubing is used; a collection bag with small, pliable tubing is easierto handle. Occasionally, an air lock will cause small tubing to stop draining;a sump port can remedy the problem. One such system is the Kendall PediatricDrainage Bag and Female Adapter.
Learn the "tricks of the trade" for catheter removal. A catheterideally is in place for no longer than a week and should be removed promptlyafter it has served its purpose.The catheter should be easy to remove. Anyresistance suggests kinking, knotting, the balloon's failure to deflate,or stone formation. Of the many tricks for dealing with a balloon failure,one of our favorites is to slide a stiff guide wire or long spinal needlein the balloon port lumen near the opening, keeping the catheter under lighttension so as to pop the balloon. Cystoscopy may be necessary to ensureevacuation of all pieces.
A kink or knot in a feeding tube can be seen on a plain film. It sometimesis possible to reduce the twisting by feeding in the catheter so the knotexpands. Insertion of a stilet may also be useful. Under no circumstancesshould the catheter be pulled out forcibly with the knot intact.
Irritative voiding symptoms following catheter removal are common. Inone study of the effects of removal of a catheter used for an imaging study,35% of pediatric patients, especially boys, had dysuria, enuresis, and hematuria.16Most of the symptoms abated after one or two days. A warm sitz bath helpedpatients who had voiding difficulties.
Familiarity breeds complacency
Although urethral catheterization has become routine, the procedure isfraught with potentially serious complications. Proper catheter selectionand gentle manipulation and placement by thoughtful, trained personnel withan appreciation of the delicate nature of the infant urethra will go a longway toward avoiding serious injury.
DR. BUKOWSKI is Director of Pediatric Urology, North Carolina Children'sHospital, Chapel Hill, NC.
DR. FREEDMAN practices pediatric urology in Los Angeles, CA.
REFERENCES
1. Prabhu S, Cochran W, Raine PAM: Postcatheterization urethral stricturesfollowing cardiac surgery in children. J Ped Surg 1985;20:69
2. Stamm WE: Catheter associated urinary tract infections: Epidemiology,pathogenesis, and prevention. Amer J of Med 1991;91(suppl 3B):65S
3. Kaplan GW, Brock WA: Urethral strictures in children. J Urol 1983;129:1200
4. Scherz HC, Kaplan GW: Etiology, diagnosis, and management of urethralstrictures in children. Urol Clin North Am 1990;17:389
5. Mitchell DJ, Parker FC: Anaphylaxis following urethral catheterization.Br J Urol 1993;71:513
6. Slater JE: Rubber anaphylaxis. NEngl J Med 1989;320:1126
7. Nacey J, Delahunt B: Re: Effect of catheter material on the incidenceof urethral strictures. Br J Urol 1992; 70:335
8. O'Brien WJ, Ryckman FC: Catheter-induced urinary bladder rupture presentingwith pneumoperitoneum. J Pediatric Surg 1994;29:1397
9. Nacey JH, Delahunt B, Tulloch AGS: The assessment of catheter-inducedurethritis using an experimental dog model. J Urol 1985;134:623
10. Litvak AS, Morris Jr JA, McRoberts JW: Normal size of the urethralmeatus in boys. J Urol 1976;115:736
11. Kroovand L: Endoscopy, in Kelalis PP, King LR, Belman AB (eds): ClinicalPediatric Urology, ed 3, vol 1. Philadelphia, PA, WB Saunders, 1992
12. Redman JF: Techniques of genital examination and bladder catheterizationin female children. Urol Clin North Am 1990;17:1
13.Foster H, Ritchey M, Bloom D: Adventitious knots in urethral catheters:Report of five cases. J Urol 1992; 148:1496
14. Konen O, Pomeranz A, Aronheim M, et al: A urethral catheter knot:A rare complication of cystourethrography. Pediatric Radiolog 1996;26:757
15. Pearson-Shaver AL, Anderson MH: Urethral catheter knots. Pediatrics1990;85:852
16. Zerin JM, Shulkin BL: Postprocedural symptoms in children who undergoimaging studies of the urinary tract: Is it the contrast material or thecatheter? Radiology 1992;182:727